Page 231 - Modern Analytical Chemistry
P. 231
1400-CH07 9/8/99 4:04 PM Page 214
214 Modern Analytical Chemistry
coefficient is unfavorable, a simple extraction will not be quantitative. Instead, the
Water-cooled
condenser extraction is accomplished by continuously passing the extracting phase through
the sample until a quantitative extraction is achieved.
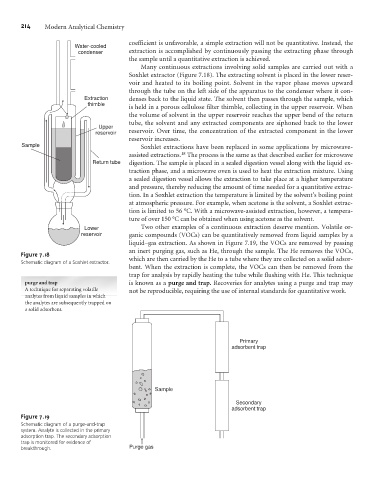
Many continuous extractions involving solid samples are carried out with a
Soxhlet extractor (Figure 7.18). The extracting solvent is placed in the lower reser-
voir and heated to its boiling point. Solvent in the vapor phase moves upward
through the tube on the left side of the apparatus to the condenser where it con-
Extraction denses back to the liquid state. The solvent then passes through the sample, which
thimble
is held in a porous cellulose filter thimble, collecting in the upper reservoir. When
the volume of solvent in the upper reservoir reaches the upper bend of the return
tube, the solvent and any extracted components are siphoned back to the lower
Upper
reservoir reservoir. Over time, the concentration of the extracted component in the lower
reservoir increases.
Sample Soxhlet extractions have been replaced in some applications by microwave-
18
assisted extractions. The process is the same as that described earlier for microwave
Return tube digestion. The sample is placed in a sealed digestion vessel along with the liquid ex-
traction phase, and a microwave oven is used to heat the extraction mixture. Using
a sealed digestion vessel allows the extraction to take place at a higher temperature
and pressure, thereby reducing the amount of time needed for a quantitative extrac-
tion. In a Soxhlet extraction the temperature is limited by the solvent’s boiling point
at atmospheric pressure. For example, when acetone is the solvent, a Soxhlet extrac-
tion is limited to 56 °C. With a microwave-assisted extraction, however, a tempera-
ture of over 150 °C can be obtained when using acetone as the solvent.
Lower Two other examples of a continuous extraction deserve mention. Volatile or-
reservoir ganic compounds (VOCs) can be quantitatively removed from liquid samples by a
liquid–gas extraction. As shown in Figure 7.19, the VOCs are removed by passing
an inert purging gas, such as He, through the sample. The He removes the VOCs,
Figure 7.18
which are then carried by the He to a tube where they are collected on a solid adsor-
Schematic diagram of a Soxhlet extractor.
bent. When the extraction is complete, the VOCs can then be removed from the
trap for analysis by rapidly heating the tube while flushing with He. This technique
purge and trap is known as a purge and trap. Recoveries for analytes using a purge and trap may
A technique for separating volatile not be reproducible, requiring the use of internal standards for quantitative work.
analytes from liquid samples in which
the analytes are subsequently trapped on
a solid adsorbent.
Primary
adsorbent trap
Sample
Secondary
adsorbent trap
Figure 7.19
Schematic diagram of a purge-and-trap
system. Analyte is collected in the primary
adsorption trap. The secondary adsorption
trap is monitored for evidence of
breakthrough. Purge gas