Page 229 - Modern Analytical Chemistry
P. 229
1400-CH07 9/8/99 4:04 PM Page 212
212 Modern Analytical Chemistry
however, if the partition coefficient is sufficiently small. If a phase containing two
extraction
The process by which a solute is solutes is brought into contact with a second phase, and K D is favorable for only one
transferred from one phase to a new of the solutes, then a separation of the solutes may be possible. The physical states of
phase. the two phases are identified when describing the separation process, with the phase
containing the sample listed first. For example, when the sample is in a liquid phase
and the second phase is a solid, the separation involves liquid–solid partitioning.
Extraction Between Two Phases When the sample is initially present in one of the
phases, the separation is known as an extraction. In a simple extraction the sample
is extracted one or more times with portions of the second phase. Simple extrac-
tions are particularly useful for separations in which only one component has a fa-
Phase 2 vorable distribution ratio. Several important separation techniques are based on
simple extractions, including liquid–liquid, liquid–solid, solid–liquid, and gas–solid
extractions.
Phase 1
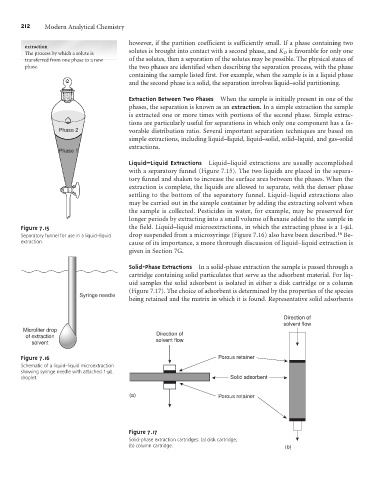
Liquid–Liquid Extractions Liquid–liquid extractions are usually accomplished
with a separatory funnel (Figure 7.15). The two liquids are placed in the separa-
tory funnel and shaken to increase the surface area between the phases. When the
extraction is complete, the liquids are allowed to separate, with the denser phase
settling to the bottom of the separatory funnel. Liquid–liquid extractions also
may be carried out in the sample container by adding the extracting solvent when
the sample is collected. Pesticides in water, for example, may be preserved for
longer periods by extracting into a small volume of hexane added to the sample in
Figure 7.15 the field. Liquid–liquid microextractions, in which the extracting phase is a 1-mL
16
Separatory funnel for use in a liquid–liquid drop suspended from a microsyringe (Figure 7.16) also have been described. Be-
extraction. cause of its importance, a more thorough discussion of liquid–liquid extraction is
given in Section 7G.
Solid-Phase Extractions In a solid-phase extraction the sample is passed through a
cartridge containing solid particulates that serve as the adsorbent material. For liq-
uid samples the solid adsorbent is isolated in either a disk cartridge or a column
(Figure 7.17). The choice of adsorbent is determined by the properties of the species
Syringe needle
being retained and the matrix in which it is found. Representative solid adsorbents
Direction of
solvent flow
Microliter drop
of extraction Direction of
solvent solvent flow
Figure 7.16 Porous retainer
Schematic of a liquid–liquid microextraction
showing syringe needle with attached 1-mL
droplet. Solid adsorbent
(a) Porous retainer
Figure 7.17
Solid-phase extraction cartridges: (a) disk cartridge;
(b) column cartridge. (b)