Page 343 - Modern Analytical Chemistry
P. 343
1400-CH09 9/9/99 2:13 PM Page 326
326 Modern Analytical Chemistry Method 9.2 Determination of Hardness of Water and Wastewater 12
Representative Methods Description of the Method. The operational definition of water hardness is the
total concentration of cations in a sample capable of forming insoluble complexes
with soap. Although most divalent and trivalent metal ions contribute to hardness,
2+
2+
and Mg . Hardness is determined by titrating with
the most important are Ca
EDTA at a buffered pH of 10. Eriochrome Black T or calmagite is used as a visual
indicator. Hardness is reported in parts per million CaCO 3 .
The photo in Colorplate 8b shows the
indicator’s color change for this titration. Procedure. Select a volume of sample requiring less than 15 mL of titrant to keep
the analysis time under 5 min and, if necessary, dilute the sample to 50 mL with
distilled water. Adjust the pH by adding 1–2 mL of a pH 10 buffer containing a small
2+
amount of Mg –EDTA. Add 1–2 drops of indicator, and titrate with a standard
solution of EDTA until the red-to-blue end point is reached.
Questions
1. Why is the sample buffered to a pH of 10? What problems might be expected
at higher or lower pHs?
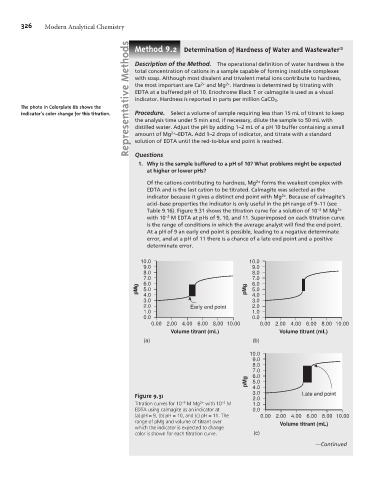
Of the cations contributing to hardness, Mg 2+ forms the weakest complex with
EDTA and is the last cation to be titrated. Calmagite was selected as the
2+
indicator because it gives a distinct end point with Mg . Because of calmagite’s
acid–base properties the indicator is only useful in the pH range of 9–11 (see
Table 9.16). Figure 9.31 shows the titration curve for a solution of 10 –3 M Mg 2+
with 10 –2 M EDTA at pHs of 9, 10, and 11. Superimposed on each titration curve
is the range of conditions in which the average analyst will find the end point.
At a pH of 9 an early end point is possible, leading to a negative determinate
error, and at a pH of 11 there is a chance of a late end point and a positive
determinate error.
10.0 10.0
9.0 9.0
8.0 8.0
7.0 7.0
pMg 6.0 pMg 6.0
5.0
5.0
4.0
3.0
3.0 4.0
2.0 Early end point 2.0
1.0 1.0
0.0 0.0
0.00 2.00 4.00 6.00 8.00 10.00 0.00 2.00 4.00 6.00 8.00 10.00
Volume titrant (mL) Volume titrant (mL)
(a) (b)
10.0
9.0
8.0
7.0
6.0
pMg 5.0
4.0
Figure 9.31 3.0 Late end point
2.0
Titration curves for 10 –3 M Mg 2+ with 10 –2 M 1.0
EDTA using calmagite as an indicator at 0.0
(a) pH = 9, (b) pH = 10, and (c) pH = 11. The 0.00 2.00 4.00 6.00 8.00 10.00
range of pMg and volume of titrant over Volume titrant (mL)
which the indicator is expected to change
color is shown for each titration curve. (c)
—Continued