Page 340 - Modern Analytical Chemistry
P. 340
1400-CH09 9/9/99 2:13 PM Page 323
Chapter 9 Titrimetric Methods of Analysis 323
The first two sensors were discussed in Section 9B.3 for acid–base titrations and are
not considered further in this section.
Finding the End Point with a Visual Indicator Most indicators for complexation
titrations are organic dyes that form stable complexes with metal ions. These dyes
are known as metallochromic indicators. To function as an indicator for an metallochromic indicator
EDTA titration, the metal–indicator complex must possess a color different from A visual indicator used to signal the end
that of the uncomplexed indicator. Furthermore, the formation constant for the point in a complexation titration.
metal–indicator complex must be less favorable than that for the metal–EDTA
complex.
m–
The indicator, In , is added to the solution of analyte, forming a colored
metal–indicator complex, MIn n-m . As EDTA is added, it reacts first with the free an-
alyte, and then displaces the analyte from the metal–indicator complex, affecting a
change in the solution’s color. The accuracy of the end point depends on the
strength of the metal–indicator complex relative to that of the metal–EDTA com-
plex. If the metal–indicator complex is too strong, the color change occurs after the
equivalence point. If the metal–indicator complex is too weak, however, the end
point is signaled before reaching the equivalence point.
Most metallochromic indicators also are weak acids or bases. The condi-
tional formation constant for the metal–indicator complex, therefore, depends on
the solution’s pH. This provides some control over the indicator’s titration error.
The apparent strength of a metal–indicator complex can be adjusted by controlling
the pH at which the titration is carried out. Unfortunately, because they also are
acid–base indicators, the color of the uncomplexed indicator changes with pH. For
example, calmagite, which we may represent as H 3 In, undergoes a change in color
–
2–
from the red of H 2 In to the blue of HIn at a pH of approximately 8.1, and from
2–
3–
the blue of HIn to the red-orange of In at a pH of approximately 12.4. Since the
color of calmagite’s metal–indicator complexes are red, it is only useful as a metal-
lochromic indicator in the pH range of 9–11, at which almost all the indicator is
2–
present as HIn .
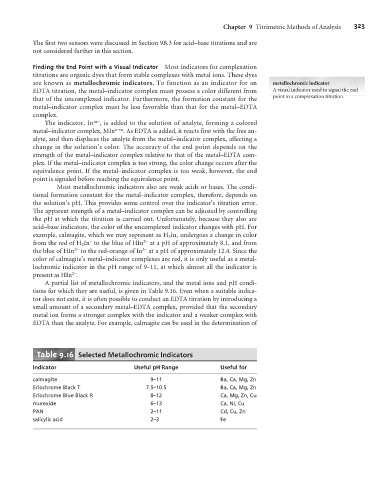
A partial list of metallochromic indicators, and the metal ions and pH condi-
tions for which they are useful, is given in Table 9.16. Even when a suitable indica-
tor does not exist, it is often possible to conduct an EDTA titration by introducing a
small amount of a secondary metal–EDTA complex, provided that the secondary
metal ion forms a stronger complex with the indicator and a weaker complex with
EDTA than the analyte. For example, calmagite can be used in the determination of
Table 9.16 Selected Metallochromic Indicators
Indicator Useful pH Range Useful for
calmagite 9–11 Ba, Ca, Mg, Zn
Eriochrome Black T 7.5–10.5 Ba, Ca, Mg, Zn
Eriochrome Blue Black R 8–12 Ca, Mg, Zn, Cu
murexide 6–13 Ca, Ni, Cu
PAN 2–11 Cd, Cu, Zn
salicylic acid 2–3 Fe