Page 339 - Modern Analytical Chemistry
P. 339
1400-CH09 9/9/99 2:13 PM Page 322
322 Modern Analytical Chemistry
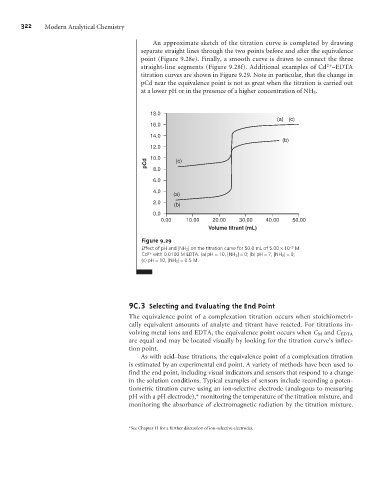
An approximate sketch of the titration curve is completed by drawing
separate straight lines through the two points before and after the equivalence
point (Figure 9.28e). Finally, a smooth curve is drawn to connect the three
2+
straight-line segments (Figure 9.28f). Additional examples of Cd –EDTA
titration curves are shown in Figure 9.29. Note in particular, that the change in
pCd near the equivalence point is not as great when the titration is carried out
at a lower pH or in the presence of a higher concentration of NH 3 .
18.0
(a) (c)
16.0
14.0
(b)
12.0
10.0
pCd 8.0 (c)
6.0
4.0
(a)
2.0 (b)
0.0
0.00 10.00 20.00 30.00 40.00 50.00
Volume titrant (mL)
Figure 9.29
Effect of pH and [NH 3 ] on the titration curve for 50.0 mL of 5.00 ´10 –3 M
Cd 2+ with 0.0100 M EDTA. (a) pH = 10, [NH 3 ] = 0; (b) pH = 7, [NH 3 ]=0;
(c) pH = 10, [NH 3 ] = 0.5 M.
9 3 Selecting and Evaluating the End Point
C.
The equivalence point of a complexation titration occurs when stoichiometri-
cally equivalent amounts of analyte and titrant have reacted. For titrations in-
volving metal ions and EDTA, the equivalence point occurs when C M and C EDTA
are equal and may be located visually by looking for the titration curve’s inflec-
tion point.
As with acid–base titrations, the equivalence point of a complexation titration
is estimated by an experimental end point. A variety of methods have been used to
find the end point, including visual indicators and sensors that respond to a change
in the solution conditions. Typical examples of sensors include recording a poten-
tiometric titration curve using an ion-selective electrode (analogous to measuring
pH with a pH electrode),* monitoring the temperature of the titration mixture, and
monitoring the absorbance of electromagnetic radiation by the titration mixture.
*See Chapter 11 for a further discussion of ion-selective electrodes.