Page 336 - Modern Analytical Chemistry
P. 336
1400-CH09 9/9/99 2:13 PM Page 319
Chapter 9 Titrimetric Methods of Analysis 319
2+
Once again, to find the [Cd ] we must account for the presence of NH 3 ; thus
2+
–9
[Cd ]= a Cd ´ Cd = (0.0881)(1.93 ´10 M) = 1.70 ´10 –10 M
C
2+
giving pCd as 9.77.
2+
After the equivalence point, EDTA is in excess, and the concentration of Cd is
2–
determined by the dissociation of the CdY complex. Examining the equation for
the complex’s conditional formation constant (equation 9.15), we see that to calcu-
2–
late C Cd we must first calculate [CdY ] and C EDTA . After adding 30.0 mL of EDTA,
these concentrations are
initial moles Cd 2 + MV
Cd Cd
[CdY 2 - ] = =
total volume V Cd +V EDTA
( . 500 ´ 10 - 3 M 500 - 3
)( . mL)
= = . 313 ´ 10 M
50.0 mL + 30.0 mL
V
moles excess EDTA M EDTA EDTA - M V Cd
Cd
C EDTA = =
total volume V Cd + V EDTA
)
(.0100 M)( .30 0 mL -(.5 00 ´ 10 -3 M)( .50 0 mL)
0
= = .625 ´ 10 -4 M
50 .0 mL + 30.0 mL
Substituting these concentrations into equation 9.15 and solving for C Cd gives
[CdY 2 - ] . 313 ´ 10 3 - M 14
= = . 894 ´ 10
CC C Cd (.625 ´ 10 - 4 )
Cd EDTA
C Cd = . 560 ´ 10 - 15 M
Thus,
2+
C
[Cd ]= a Cd ´ Cd = (0.0881)(5.60 ´10 –15 M) = 4.93 ´10 –16 M
2+
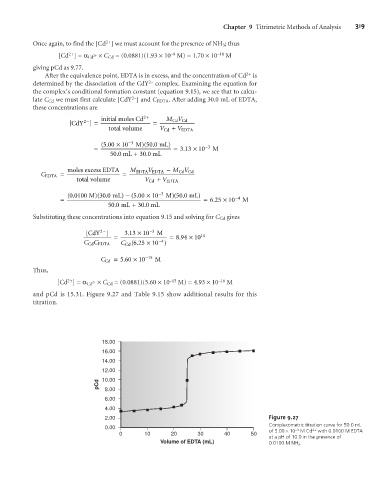
and pCd is 15.31. Figure 9.27 and Table 9.15 show additional results for this
titration.
18.00
16.00
14.00
12.00
pCd 10.00
8.00
6.00
4.00
2.00 Figure 9.27
Complexometric titration curve for 50.0 mL
0.00 –3 2+
0 10 20 30 40 50 of 5.00 ´10 M Cd with 0.0100 M EDTA
at a pH of 10.0 in the presence of
Volume of EDTA (mL) 0.0100 M NH 3 .