Page 331 - Modern Analytical Chemistry
P. 331
1400-CH09 9/9/99 2:13 PM Page 314
314 Modern Analytical Chemistry
14.0
12.0
10.0
8.0
pH
6.0
4.0
2.0
0.0
0.00 20.00 40.00 60.00 80.00 100.00 120.00 140.00
Volume of titrant
(a)
14.0
12.0
10.0
8.0
pH
6.0
4.0
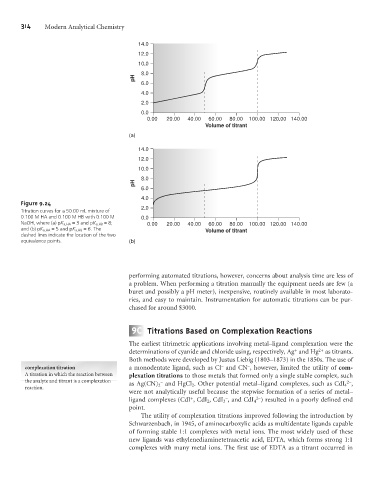
Figure 9.24
2.0
Titration curves for a 50.00 mL mixture of
0.100 M HA and 0.100 M HB with 0.100 M 0.0
NaOH, where (a) pK a,HA = 3 and pK a,HB =8; 0.00 20.00 40.00 60.00 80.00 100.00 120.00 140.00
and (b) pK a,HA = 5 and pK a,HB = 6. The Volume of titrant
dashed lines indicate the location of the two
equivalence points. (b)
performing automated titrations, however, concerns about analysis time are less of
a problem. When performing a titration manually the equipment needs are few (a
buret and possibly a pH meter), inexpensive, routinely available in most laborato-
ries, and easy to maintain. Instrumentation for automatic titrations can be pur-
chased for around $3000.
9 C Titrations Based on Complexation Reactions
The earliest titrimetric applications involving metal–ligand complexation were the
2+
+
determinations of cyanide and chloride using, respectively, Ag and Hg as titrants.
Both methods were developed by Justus Liebig (1803–1873) in the 1850s. The use of
–
–
complexation titration a monodentate ligand, such as Cl and CN , however, limited the utility of com-
A titration in which the reaction between plexation titrations to those metals that formed only a single stable complex, such
the analyte and titrant is a complexation – 2–
as Ag(CN) 2 and HgCl 2 . Other potential metal–ligand complexes, such as CdI 4 ,
reaction.
were not analytically useful because the stepwise formation of a series of metal–
–
+
2–
ligand complexes (CdI , CdI 2 , CdI 3 , and CdI 4 ) resulted in a poorly defined end
point.
The utility of complexation titrations improved following the introduction by
Schwarzenbach, in 1945, of aminocarboxylic acids as multidentate ligands capable
of forming stable 1:1 complexes with metal ions. The most widely used of these
new ligands was ethylenediaminetetraacetic acid, EDTA, which forms strong 1:1
complexes with many metal ions. The first use of EDTA as a titrant occurred in