Page 58 - MODERN ASPECTS OF ELECTROCHEMISTRY
P. 58
45
Voltaic Cells in Electrochemistry and Surface Chemistry of Liquids
liquid, which must take into account the molecular nature of a liquid. 21
There is some evidence that the surface potential of very thin layers of
water covering metal surfaces, for instance emersed electrodes, has a
negative value. 158
The published experimental estimates of the surface potentials of
various organic solvents have been derived mainly from the data on the
real, asi, and chemical, µSi, energies of solvation of ions:
S
Fχ =α i µ S i
S
or from the differences of these energies in the solvent and water 8187
S
S
S
F∆ χ =∆ w α i ∆ w µ i (32)
w
According to the above equations, the surface potential is the difference
between the real and chemical potentials of charged particles dissolved in
the liquid phase.
Trasatti has calculated the potentials (χ ) of several organic. solvents
S
from Volta potentials and the partial surface potentials on the mercury
solution phase boundaries at the potential of zero charge. 79
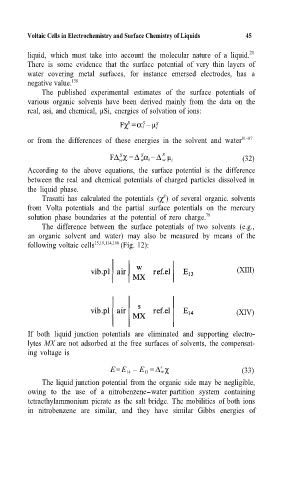
The difference between the surface potentials of two solvents (e.g.,
an organic solvent and water) may also be measured by means of the
following voltaic cells 15,19,114,160 (Fig. 12):
(XIII)
(XIV)
If both liquid junction potentials are eliminated and supporting electro-
lytes MX are not adsorbed at the free surfaces of solvents, the compensat-
ing voltage is
s
E=E E = ∆ wχ (33)
14
13
The liquid junction potential from the organic side may be negligible,
owing to the use of a nitrobenzene-water partition system containing
tetraethylammonium picrate as the salt bridge. The mobilities of both ions
in nitrobenzene are similar, and they have similar Gibbs energies of