Page 59 - MODERN ASPECTS OF ELECTROCHEMISTRY
P. 59
Zbigniew KoczorowskiA
46
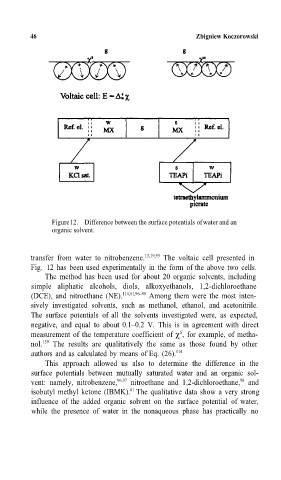
Figure 12. Difference between the surface potentials of water and an
organic solvent.
transfer from water to nitrobenzene. 15,19,93 The voltaic cell presented in
Fig. 12 has been used experimentally in the form of the above two cells.
The method has been used for about 20 organic solvents, including
simple aliphatic alcohols, diols, alkoxyethanols, 1,2-dichloroethane
(DCE), and nitroethane (NE). 114,61,9698 Among them were the most inten-
sively investigated solvents, such as methanol, ethanol, and acetonitrile.
The surface potentials of all the solvents investigated were, as expected,
negative, and equal to about 0.10.2 V. This is in agreement with direct
S
measurement of the temperature coefficient of χ , for example, of metha-
159
nol. The results are qualitatively the same as those found by other
authors and as calculated by means of Eq. (26). 114
This approach allowed us also to determine the difference in the
surface potentials between mutually saturated water and an organic sol-
98
vent: namely, nitrobenzene, 96,97 nitroethane and 1,2-dichloroethane, and
61
isobutyl methyl ketone (IBMK). The qualitative data show a very strong
influence of the added organic solvent on the surface potential of water,
while the presence of water in the nonaqueous phase has practically no