Page 60 - MODERN ASPECTS OF ELECTROCHEMISTRY
P. 60
47
Voltaic Cells in Electrochemistry and Surface Chemistry of LiquidsA
S
effect on its χ potential. In the case of NB, NE, and DCE solvents, the
∆ w+s values for the water phase saturated with these solvents are lower
wχ
s
than ∆wχ .For IBMK, the maximum is observed, which means that the
surface orientation of the molecules of the solvent in water is larger than
in pure IBMK. The information resulting from the surface potential
measurements may be used in the analysis of the interfacial structure of
24
liquid/liquid interfaces and their dipole and zero-charge potentials. Also,
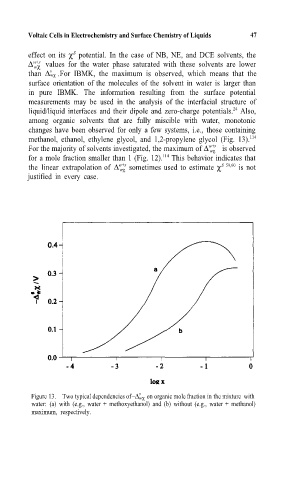
among organic solvents that are fully miscible with water, monotonic
changes have been observed for only a few systems, i.e., those containing
methanol, ethanol, ethylene glycol, and 1,2-propylene glycol (Fig. 13). 114
w+s
For the majority of solvents investigated, the maximum of ∆ wχ is observed
for a mole fraction smaller than 1 (Fig. 12). This behavior indicates that
114
the linear extrapolation of ∆ wχ sometimes used to estimate χ S 59,60 is not
w+s
justified in every case.
s
Figure 13. Two typical dependencies of∆ wχ on organic mole fraction in the mixture with
water: (a) with (e.g., water + methoxyethanol) and (b) without (e.g., water + methanol)
maximum, respectively.