Page 240 - Modern Derivatization Methods for Separation Sciences
P. 240
Document Página 1 de 1
Page 110
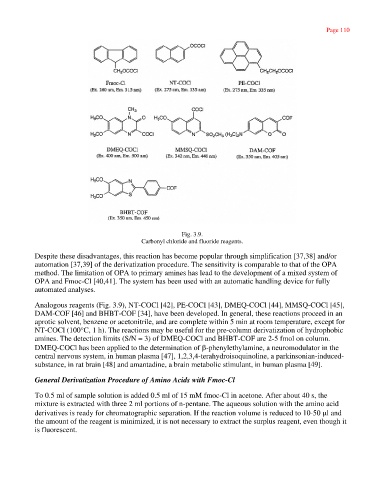
Fig. 3.9.
Carbonyl chloride and fluoride reagents.
Despite these disadvantages, this reaction has become popular through simplification [37,38] and/or
automation [37,39] of the derivatization procedure. The sensitivity is comparable to that of the OPA
method. The limitation of OPA to primary amines has lead to the development of a mixed system of
OPA and Fmoc-Cl [40,41]. The system has been used with an automatic handling device for fully
automated analyses.
Analogous reagents (Fig. 3.9), NT-COCl [42], PE-COCI [43], DMEQ-COCl [44], MMSQ-COCl [45],
DAM-COF [46] and BHBT-COF [34], have been developed. In general, these reactions proceed in an
aprotic solvent, benzene or acetonitrile, and are complete within 5 min at room temperature, except for
NT-COCl (100°C, 1 h). The reactions may be useful for the pre-column derivatization of hydrophobic
amines. The detection limits (S/N = 3) of DMEQ-COCl and BHBT-COF are 2-5 fmol on column.
DMEQ-COCl has been applied to the determination of β-phenylethylamine, a neuromodulator in the
central nervous system, in human plasma [47], 1,2,3,4-terahydroisoquinoline, a parkinsonian-induced-
substance, in rat brain [48] and amantadine, a brain metabolic stimulant, in human plasma [49].
General Derivatization Procedure of Amino Acids with Fmoc-Cl
To 0.5 ml of sample solution is added 0.5 ml of 15 mM fmoc-Cl in acetone. After about 40 s, the
mixture is extracted with three 2 ml portions of n-pentane. The aqueous solution with the amino acid
derivatives is ready for chromatographic separation. If the reaction volume is reduced to 10-50 µl and
the amount of the reagent is minimized, it is not necessary to extract the surplus reagent, even though it
is fluorescent.
http://emedia.netlibrary.com/nlreader/nlreader.dll?bookid=17968&filename=Page_110.ht... 30/09/2003