Page 32 - Modern Derivatization Methods for Separation Sciences
P. 32
Document Página 1 de 1
Page 5
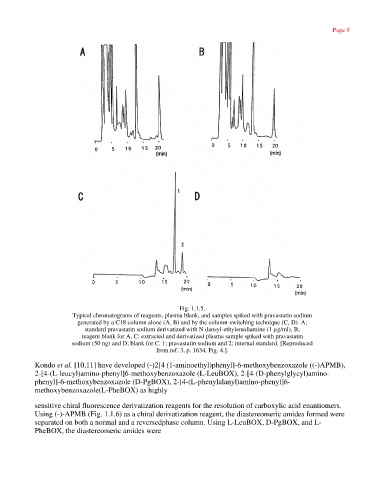
Fig. 1.1.5.
Typical chromatograms of reagents, plasma blank, and samples spiked with pravastatin sodium
generated by a C18 column alone (A, B) and by the column-switching technique (C, D). A;
standard pravastatin sodium derivatized with N dansyl-ethylenediamine (1 µg/ml), B;
reagent blank for A, C; extracted and derivatized plasma sample spiked with pravastatin
sodium (50 ng) and D; blank for C. 1; pravastatin sodium and 2; internal standard. [Reproduced
from ref. 3, p. 1634, Fig. 4.].
Kondo et al. [10,11] have developed (-)2[4 (1-aminoethyl)phenyl]-6-methoxybenzoxazole ((-)APMB),
2-[4-(L-leucyl)amino-phenyl]6-methoxybenzoxazole (L-LeuBOX), 2-[4-(D-phenylglycyl)amino-
phenyl]-6-methoxybenzoxazole (D-PgBOX), 2-[4-(L-phenylalanyl)amino-phenyl]6-
methoxybenzoxazole(L-PheBOX) as highly
sensitive chiral fluorescence derivatization reagents for the resolution of carboxylic acid enantiomers.
Using (-)-APMB (Fig. 1.1.6) as a chiral derivatization reagent, the diastereomeric amides formed were
separated on both a normal and a reversedphase column. Using L-LeuBOX, D-PgBOX, and L-
PheBOX, the diastereomeric amides were
http://emedia.netlibrary.com/nlreader/nlreader.dll?bookid=17968&filename=Page_5.html 30/09/2003