Page 33 - Modern Derivatization Methods for Separation Sciences
P. 33
Document Página 1 de 2
Page 6
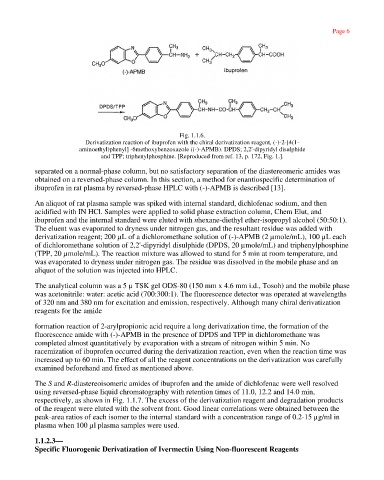
Fig. 1.1.6.
Derivatization reaction of ibuprofen with the chiral derivatization reagent, (-)-2-[4(1-
aminoethyl)phenyl] -6methoxybenzoxazole ((-)-APMB). DPDS; 2,2'-dipyridyl disulphide
and TPP; triphenylphosphine. [Reproduced from ref. 13, p. 172, Fig. 1.].
separated on a normal-phase column, but no satisfactory separation of the diastereomeric amides was
obtained on a reversed-phase column. In this section, a method for enantiospecific determination of
ibuprofen in rat plasma by reversed-phase HPLC with (-)-APMB is described [13].
An aliquot of rat plasma sample was spiked with internal standard, dichlofenac sodium, and then
acidified with IN HCl. Samples were applied to solid phase extraction column, Chem Elut, and
ibuprofen and the internal standard were eluted with nhexane-diethyl ether-isopropyl alcohol (50:50:1).
The eluent was evaporated to dryness under nitrogen gas, and the resultant residue was added with
derivatization reagent; 200 µL of a dichloromethane solution of (-)-APMB (2 µmole/mL), 100 µL each
of dichloromethane solution of 2,2'-dipyridyl disulphide (DPDS, 20 µmole/mL) and triphenylphosphine
(TPP, 20 µmole/mL). The reaction mixture was allowed to stand for 5 min at room temperature, and
was evaporated to dryness under nitrogen gas. The residue was dissolved in the mobile phase and an
aliquot of the solution was injected into HPLC.
The analytical column was a 5 µ TSK gel ODS-80 (150 mm x 4.6 mm i.d., Tosoh) and the mobile phase
was acetonitrile: water: acetic acid (700:300:1). The fluorescence detector was operated at wavelengths
of 320 nm and 380 nm for excitation and emission, respectively. Although many chiral derivatization
reagents for the amide
formation reaction of 2-arylpropionic acid require a long derivatization time, the formation of the
fluorescence amide with (-)-APMB in the presence of DPDS and TPP in dichloromethane was
completed almost quantitatively by evaporation with a stream of nitrogen within 5 min. No
racemization of ibuprofen occurred during the derivatization reaction, even when the reaction time was
increased up to 60 min. The effect of all the reagent concentrations on the derivatization was carefully
examined beforehand and fixed as mentioned above.
The S and R-diastereoisomeric amides of ibuprofen and the amide of dichlofenac were well resolved
using reversed-phase liquid chromatography with retention times of 11.0, 12.2 and 14.0 min,
respectively, as shown in Fig. 1.1.7. The excess of the derivatization reagent and degradation products
of the reagent were eluted with the solvent front. Good linear correlations were obtained between the
peak-area ratios of each isomer to the internal standard with a concentration range of 0.2-15 µg/ml in
plasma when 100 µl plasma samples were used.
1.1.2.3—
Specific Fluorogenic Derivatization of Ivermectin Using Non-fluorescent Reagents
http://emedia.netlibrary.com/nlreader/nlreader.dll?bookid=17968&filename=Page_6.html 30/09/2003