Page 120 - Modern physical chemistry
P. 120
6.4 Solutions 111
VII
P(atm)
-40 o 40
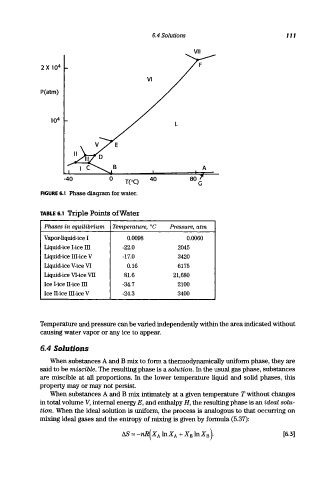
FIGURE 6.1 Phase diagram for water.
TABLE 6.1 Triple Points of Water
Phases in equilibrium Temperature, °C Pressure, atm
Vapor-liquid-ice I 0.0098 0.0060
Liquid-ice I-ice III -22.0 2045
Liquid-ice III-ice V -17.0 3420
Liquid-ice V-ice VI 0.16 6175
Liquid-ice VI-ice VII 81.6 21,680
Ice I-ice IT-ice III -34.7 2100
Ice IT-ice III-ice V -24.3 3400
Temperature and pressure can be varied independently within the area indicated without
causing water vapor or any ice to appear.
6.4 Solutions
When substances A and B mix to fonn a thennodynamically unifonn phase, they are
said to be miscible. The resulting phase is a solution. In the usual gas phase, substances
are miscible at all proportions. In the lower temperature liquid and solid phases, this
property mayor may not persist.
When substances A and B mix intimately at a given temperature T without changes
in total volume V, internal energy E, and enthalpy H, the resulting phase is an ideal solu-
tion. When the ideal solution is unifonn, the process is analogous to that occurring on
mixing ideal gases and the entropy of mixing is given by fonnula (5.37):
M' = -nR(XA lnXA +XB lnXB)' [6.3]