Page 124 - Modern physical chemistry
P. 124
6.6 Singularities Separating Phases 115
leads to fractionation when the L curve is reached. The low temperature phase then
moves towards the maximum. When this point is reached, the resulting high tempera-
ture phase has the same composition as the low temperature one and transition contin-
ues as for a pure substance.
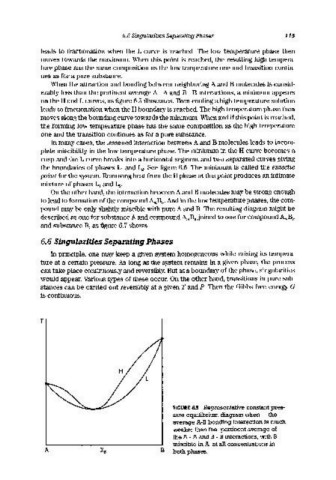
When the attraction and bonding between neighboring A and B molecules is consid-
erably less than the pertinent average A - A and B - B interactions, a minimum appears
on the H and L curves, as figure 6.5 illustrates. Then cooling a high temperature solution
leads to fractionation when the H boundary is reached. The high temperature phase then
moves along the bounding curve towards the minimum. When and if this point is reached,
the forming low temperature phase has the same composition as the high temperature
one and the transition continues as for a pure substance.
In many cases, the lessened interaction between A and B molecules leads to incom-
plete miscibility in the low temperature phase. The minimum in the H curve becomes a
cusp and the L curve breaks into a horizontal segment and two separated curves giving
the boundaries of phases Ll and L 2 • See figure 6.6. The minimum is called the eutectic
point for the system. Removing heat from the H phase at this point produces an intimate
mixture of phases Ll and ~.
On the other hand, the interaction between A and B molecules may be strong enough
to lead to formation of the compound A."Bn- And in the low temperature phases, the com-
pound may be only slightly miscible with pure A and B. The resulting diagram might be
described as one for substance A and compound A."B n joined to one for compound A."Bn
and substance B, as figure 6.7 shows.
6.6 Singularities Separating Phases
In principle, one may keep a given system homogeneous while raising its tempera-
ture at a certain pressure. As long as the system remains in a given phase, the process
can take place continuously and reversibly. But at a boundary of the phase, singularities
would appear. Various types of these occur. On the other hand, transitions in pure sub-
stances can be carried out reversibly at a given T and P. Then the Gibbs free energy G
is continuous.
FIGURE 6.5 Representative constant pres-
sure equilibrium diagram when the
average A-B bonding interaction is much
weaker than the pertinent average of
the A - A and B - B interactions, with B
miscible in A at all concentrations in
both phases.