Page 126 - Modern physical chemistry
P. 126
6.6 Singularities Separating Phases 117
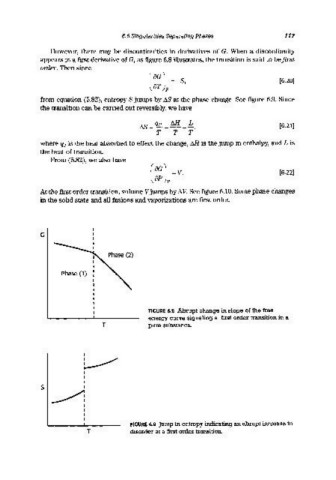
However, there may be discontinuities in derivatives of G. When a discontinuity
appears in a first derivative of G, as figure 6.8 illustrates, the transition is said to befirst
order. Then since
(~~Jp =-8, [6.20]
from equation (5.82), entropy 8 jumps by D.S at the phase change. See figure 6.9. Since
the transition can be carried out reversibly, we have
D.S _ qp _ Ml _ L [6.21]
-T-T-r'
where qp is the heat absorbed to effect the change, Ml is the jump in enthalpy, and L is
the heat of transition.
From (5.82), we also have
[~~l =V. [6.22]
At the first order transition, volume V jumps by ~ V. See figure 6.10. Some phase changes
in the solid state and all fusions and vaporizations are first order.
G
Phase (1)
FIGURE 6.8 Abrupt change in slope of the free
energy curve signaling a first order transition in a
T pure substance.
I
~
I
I
I
5 I
~
I
I
I
I
FIGURE 6.9 Jump in entropy indicating an abrupt increase in
T disorder at a first order transition.