Page 127 - Modern physical chemistry
P. 127
118 Relationships between Phases
V
I
I
V ......-ol
I
I
I
I
I
I
I
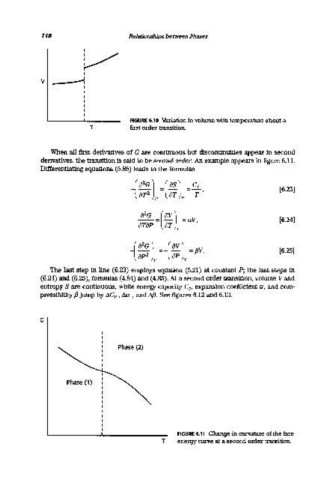
FIGURE 6.10 Variation in volume with temperature about a
T first order transition.
When all first derivatives of G are continuous but discontinuities appear in second
derivatives, the transition is said to be second order. An example appears in figure 6.11.
Differentiating equations (5.85) leads to the fomlUlas
{:~~l ~[:)p <;, [6.23]
2
aG _[av) -aV [6.24]
aTap - aT p - ,
~[:~ 1 ~~[:;l ~pv. [6.25]
The last step in line (6.23) employs equation (5.21) at constant P; the last steps in
(6.24) and (6.25), formulas (4.84) and (4.85). At a second order transition, volume V and
entropy S are continuous, while energy capacity Cp, expansion coefficient a, and com-
pressibility f3 jump by /lCp , Aa , and A{3. See figures 6.12 and 6.13.
G
phase (2)
Phase (1)
FIGURE 6.11 Change in curvature of the free
T energy curve at a second order transition.