Page 161 - Modern physical chemistry
P. 161
1.10 Partial Molar Gibbs Energy 153
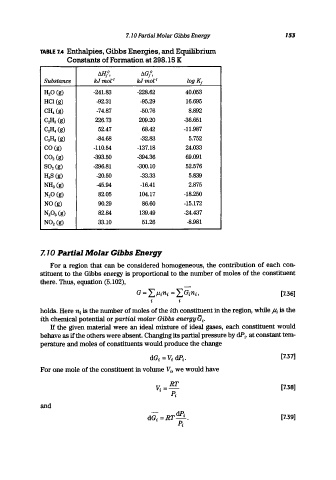
TABLE 7.4 Enthalpies, Gibbs Energies, and Equilibrium
Constants of Formation at 298.16 K
Ml/, 6G/,
Substance kJmo[-I kJ mol- I logK f
H 20 (g) -241.83 -228.62 40.053
HCl(g) -92.31 -95.29 16.695
CH 4 (g) -74.87 -50.76 8.892
C 2H 2 (g) 226.73 209.20 -36.651
C 2H 4 (g) 52.47 68.42 -11.987
C 21ia (g) -84.68 -32.83 5.752
CO (g) -110.54 -137.18 24.033
CO 2 (g) -393.50 -394.36 69.091
802 (g) -296.81 -300.10 52.576
H 28 (g) -20.50 -33.33 5.839
NHa (g) -45.94 -16.41 2.875
N 20 (g) 82.05 104.17 -18.250
NO (g) 90.29 86.60 -15.172
N 20 a (g) 82.84 139.49 -24.437
N0 2 (g) 33.10 51.26 -8.981
Z 10 Partial Molar Gibbs Energy
For a region that can be considered homogeneous, the contribution of each con-
stituent to the Gibbs energy is proportional to the number of moles of the constituent
there. Thus, equation (5.102),
G = L,uini = LGini, [7.36]
i i
holds. Here n i is the number of moles of the ith constituent in the region, while i4 is the
ith chemical potential or partial molar Gibbs energyG i •
If the given material were an ideal mixture of ideal gases, each constituent would
behave as if the others were absent. Changing its partial pressure by dP i • at constant tem-
perature and moles of constituents would produce the change
[7.37]
dGi = Vi dP i ·
For one mole of the constituent in volume Vi' we would have
[7.38]
and
[7.39]