Page 192 - Modern physical chemistry
P. 192
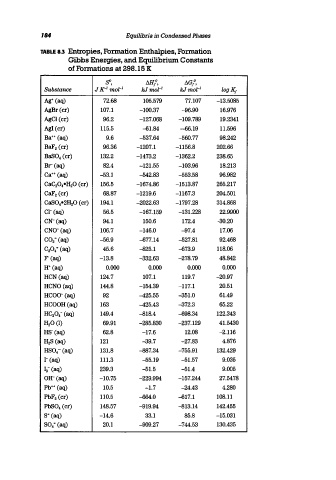
184 Equilibria in Condensed Phases
TABLE 8.3 Entropies. Formation Enthalpies. Formation
Gibbs Energies. and Equilibrium Constants
of Formations at 298.15 K
so, M/, IlG/,
Substance J K- 1 mol- I kJmor i kJmol- I logK f
Ag+ (aq) 72.68 105.579 77.107 -13.5085
AgBr(cr) 107.1 -100.37 -96.90 16.976
AgCl(cr) 96.2 -127.068 -109.789 19.2341
AgI (cr) 115.5 --61.84 -66.19 11.596
Ba++ (aq) 9.6 -537.64 -560.77 98.242
BaF 2 (cr) 96.36 -1207.1 -1156.8 202.66
BaS0 4 (cr) 132.2 -1473.2 -1362.2 238.65
Br(aq) 82.4 -121.55 -103.96 18.213
Ca++ (aq) -53.1 -542.83 -553.58 96.982
CaC 20 4-H 20 (cr) 156.5 -1674.86 -1513.87 265.217
CaF 2 (cr) 68.87 -1219.6 -1167.3 204.501
CaS0 4-2H 20 (cr) 194.1 -2022.63 -1797.28 314.868
Cl- (aq) 56.5 -167.159 -131.228 22.9900
CN-(aq) 94.1 150.6 172.4 -30.20
CNO-(aq) 106.7 -146.0 -97.4 17.06
COaC (aq) -56.9 --677.14 -527.81 92.468
C 20 4 ' (aq) 45.6 -825.1 --673.9 118.06
P(aq) -13.8 -332.63 -278.79 48.842
H+ (aq) 0.000 0.000 0.000 0.000
HCN (aq) 124.7 107.1 119.7 -20.97
HCNO(aq) 144.8 -154.39 -117.1 20.51
HCOO-(aq) 92 -425.55 -351.0 61.49
HCOOH(aq) 163 -425.43 -372.3 65.22
HC 20 4-(aq) 149.4 -818.4 --698.34 122.343
H2°(l) 69.91 -285.830 -237.129 41.5430
HS·(aq) 62.8 -17.6 12.08 -2.116
H 28 (aq) 121 -39.7 -27.83 4.876
HS0 4 - (aq) 131.8 -887.34 -755.91 132.429
1- (aq) 111.3 -55.19 -51.57 9.035
la- (aq) 239.3 -51.5 -51.4 9.005
OH- (aq) -10.75 -229.994 -157.244 27.5478
Pb++ (aq) 10.5 -1.7 -24.43 4.280
PbF 2 (cr) 110.5 --664.0 --617.1 108.11
Pb80 4 (cr) 148.57 -919.94 -813.14 142.455
8= (aq) -14.6 33.1 85.8 -15.031
80 4 - (aq) 20.1 -909.27 -744.53 130.435