Page 59 - Modern physical chemistry
P. 59
48 Gases and Collective Properties
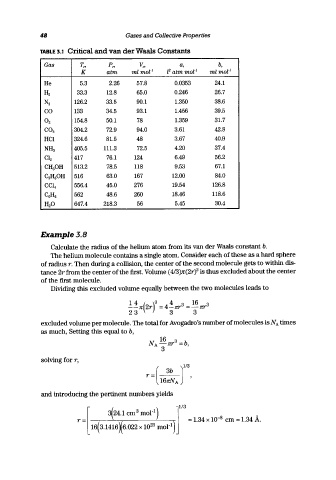
TABLE 3.1 Critical and van der Waals Constants
Gas T", P", v", a, b,
K atm ml mol- 1 l2 atm mor l ml mor l
He 5.3 2.26 57.8 0.0353 24.1
H2 33.3 12.8 65.0 0.246 26.7
N2 126.2 33.5 90.1 1.350 38.6
CO 133 34.5 93.1 1.456 39.5
O2 154.8 50.1 78 1.359 31.7
CO2 304.2 72.9 94.0 3.61 42.8
HCI 324.6 81.5 48 3.67 40.9
NH3 405.5 111.3 72.5 4.20 37.4
Cl2 417 76.1 124 6.49 56.2
CH3 0H 513.2 78.5 118 9.53 67.1
C2 HsOH 516 63.0 167 12.00 84.0
CCl 4 556.4 45.0 276 19.54 126.8
C6H6 562 48.6 260 18.46 118.6
H2 O 647.4 218.3 56 5.45 30.4
£Xample3.B
Calculate the radius of the helium atom from its van der Waals constant b.
The helium molecule contains a single atom. Consider each of these as a hard sphere
of radius r. Then during a collision, the center of the second molecule gets to within dis-
tance 2r from the center of the first. Volume (413)Jl"(2rY is thus excluded about the center
of the first molecule.
Dividing this excluded volume equally between the two molecules leads to
.!in(2r)3 = 4inr3 = 16 nr3
23 3 3
excluded volume per molecule. The total for Avogadro's number of molecules is NA times
as much, Setting this equal to b,
solving for r,
r=(16:J,
and introducing the pertinent numbers yields
113
'l{24.1 cm 3 mOl-I)
r = [ \, ] = 1.34 x 10-8 em = 1.34 A.
16( 3.1416 X 6.022 x 10 23 mOl-I)