Page 61 - Modern physical chemistry
P. 61
50 Gases and Collective Properties
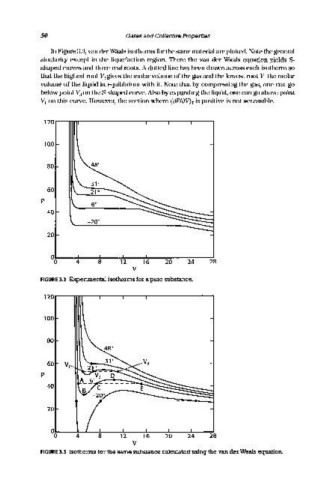
In Figure 3.3, van der Waals isotherms for the same material are plotted. Note the general
similarity except in the liquefaction region. There the van der Waals equation yields S-
shaped curves and three real roots. A dotted line has been drawn across each isotherm so
that the highest root V3 gives the molar volume of the gas and the lowest root VI the molar
volume of the liquid in equilibrium with it. Note that by compressing the gas, one can go
below point Va on the S-shaped curve. Also by expanding the liquid, one can go above point
VI on this curve. However, the section where cap/en')r is positive is not accessible.
120
100
80
60
P
40
20
0
0 4 8 12 16 20 24 28
v
FIGURE 3.2 Experimental isotherms for a pure substance.
120~--~n---~----~----~----~----~--~
100
80
60 V 1
P
40
20
0
0 16 28
V
FIGURE 3.3 Isotherms for the same substance calculated using the van der Waals equation.