Page 161 - Multidimensional Chromatography
P. 161
Unified Chromatography 153
7.2 THE PHASE DIAGRAM VIEW OF
UNIFIED CHROMATOGRAPHY
We must start with fluid behavior to understand the basic concepts of unified chro-
matography. We must forget most of what we know from common experience about
liquid and gas behavior since this experience is tied with ambient conditions.
Instead, we must embrace the new possibilities afforded by temperatures and pres-
sures that are different from ambient. This new view requires phase diagrams
(17, 18).
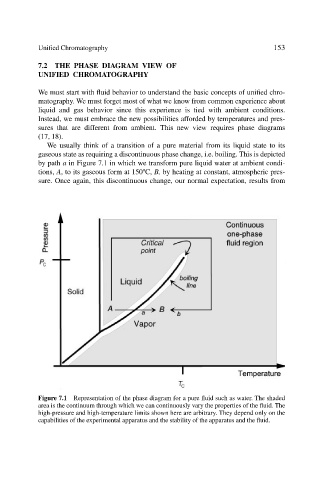
We usually think of a transition of a pure material from its liquid state to its
gaseous state as requiring a discontinuous phase change, i.e. boiling. This is depicted
by path a in Figure 7.1 in which we transform pure liquid water at ambient condi-
o
tions, A, to its gaseous form at 150 C, B, by heating at constant, atmospheric pres-
sure. Once again, this discontinuous change, our normal expectation, results from
Figure 7.1 Representation of the phase diagram for a pure fluid such as water. The shaded
area is the continuum through which we can continuously vary the properties of the fluid. The
high-pressure and high-temperature limits shown here are arbitrary. They depend only on the
capabilities of the experimental apparatus and the stability of the apparatus and the fluid.