Page 163 - Multidimensional Chromatography
P. 163
Unified Chromatography 155
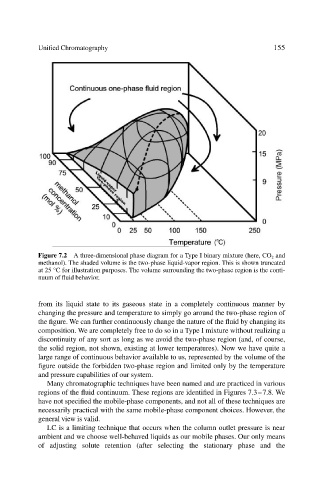
Figure 7.2 A three-dimensional phase diagram for a Type I binary mixture (here, CO 2 and
methanol). The shaded volume is the two-phase liquid-vapor region. This is shown truncated
at 25 °C for illustration purposes. The volume surrounding the two-phase region is the conti-
nuum of fluid behavior.
from its liquid state to its gaseous state in a completely continuous manner by
changing the pressure and temperature to simply go around the two-phase region of
the figure. We can further continuously change the nature of the fluid by changing its
composition. We are completely free to do so in a Type I mixture without realizing a
discontinuity of any sort as long as we avoid the two-phase region (and, of course,
the solid region, not shown, existing at lower temperatures). Now we have quite a
large range of continuous behavior available to us, represented by the volume of the
figure outside the forbidden two-phase region and limited only by the temperature
and pressure capabilities of our system.
Many chromatographic techniques have been named and are practiced in various
regions of the fluid continuum. These regions are identified in Figures 7.3–7.8. We
have not specified the mobile-phase components, and not all of these techniques are
necessarily practical with the same mobile-phase component choices. However, the
general view is valid.
LC is a limiting technique that occurs when the column outlet pressure is near
ambient and we choose well-behaved liquids as our mobile phases. Our only means
of adjusting solute retention (after selecting the stationary phase and the