Page 164 - Multidimensional Chromatography
P. 164
156 Multidimensional Chromatography
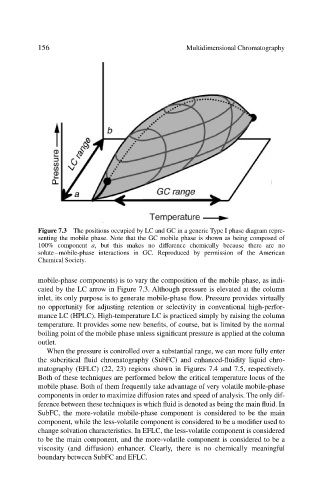
Figure 7.3 The positions occupied by LC and GC in a generic Type I phase diagram repre-
senting the mobile phase. Note that the GC mobile phase is shown as being composed of
100% component a, but this makes no difference chemically because there are no
solute–mobile-phase interactions in GC. Reproduced by permission of the American
Chemical Society.
mobile-phase components) is to vary the composition of the mobile phase, as indi-
cated by the LC arrow in Figure 7.3. Although pressure is elevated at the column
inlet, its only purpose is to generate mobile-phase flow. Pressure provides virtually
no opportunity for adjusting retention or selectivity in conventional high-perfor-
mance LC (HPLC). High-temperature LC is practiced simply by raising the column
temperature. It provides some new benefits, of course, but is limited by the normal
boiling point of the mobile phase unless significant pressure is applied at the column
outlet.
When the pressure is controlled over a substantial range, we can more fully enter
the subcritical fluid chromatography (SubFC) and enhanced-fluidity liquid chro-
matography (EFLC) (22, 23) regions shown in Figures 7.4 and 7.5, respectively.
Both of these techniques are performed below the critical temperature locus of the
mobile phase. Both of them frequently take advantage of very volatile mobile-phase
components in order to maximize diffusion rates and speed of analysis. The only dif-
ference between these techniques is which fluid is denoted as being the main fluid. In
SubFC, the more-volatile mobile-phase component is considered to be the main
component, while the less-volatile component is considered to be a modifier used to
change solvation characteristics. In EFLC, the less-volatile component is considered
to be the main component, and the more-volatile component is considered to be a
viscosity (and diffusion) enhancer. Clearly, there is no chemically meaningful
boundary between SubFC and EFLC.