Page 166 - Multidimensional Chromatography
P. 166
158 Multidimensional Chromatography
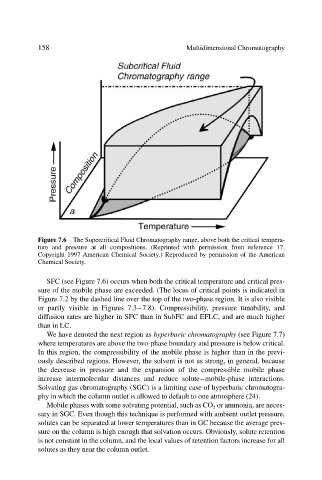
Figure 7.6 The Supercritical Fluid Chromatography range, above both the critical tempera-
ture and pressure at all compositions. (Reprinted with permission from reference 17.
Copyright 1997 American Chemical Society.) Reproduced by permission of the American
Chemical Society.
SFC (see Figure 7.6) occurs when both the critical temperature and critical pres-
sure of the mobile phase are exceeded. (The locus of critical points is indicated in
Figure 7.2 by the dashed line over the top of the two-phase region. It is also visible
or partly visible in Figures 7.3–7.8). Compressibility, pressure tunability, and
diffusion rates are higher in SFC than in SubFC and EFLC, and are much higher
than in LC.
We have denoted the next region as hyperbaric chromatography (see Figure 7.7)
where temperatures are above the two-phase boundary and pressure is below critical.
In this region, the compressibility of the mobile phase is higher than in the previ-
ously described regions. However, the solvent is not as strong, in general, because
the decrease in pressure and the expansion of the compressible mobile phase
increase intermolecular distances and reduce solute–mobile-phase interactions.
Solvating gas chromatography (SGC) is a limiting case of hyperbaric chromatogra-
phy in which the column outlet is allowed to default to one atmosphere (24).
Mobile phases with some solvating potential, such as CO 2 or ammonia, are neces-
sary in SGC. Even though this technique is performed with ambient outlet pressure,
solutes can be separated at lower temperatures than in GC because the average pres-
sure on the column is high enough that solvation occurs. Obviously, solute retention
is not constant in the column, and the local values of retention factors increase for all
solutes as they near the column outlet.