Page 268 - Multidimensional Chromatography
P. 268
Biomedical and Pharmaceutical Applications 263
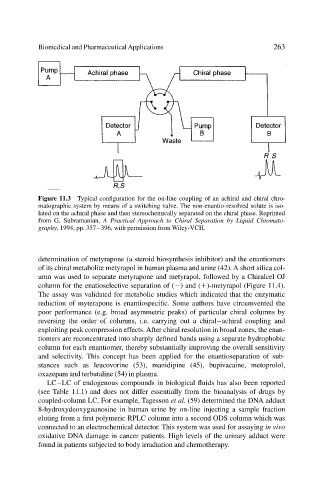
Figure 11.3 Typical configuration for the on-line coupling of an achiral and chiral chro-
matographic system by means of a switching valve. The non-enantio-resolved solute is iso-
lated on the achiral phase and then stereochemically separated on the chiral phase. Reprinted
from G. Subramanian, A Practical Approach to Chiral Separation by Liquid Chromato-
graphy, 1994, pp. 357–396, with permission from Wiley-VCH.
determination of metyrapone (a steroid biosynthesis inhibitor) and the enantiomers
of its chiral metabolite metyrapol in human plasma and urine (42). A short silica col-
umn was used to separate metyrapone and metyrapol, followed by a Chiralcel OJ
column for the enatioselective separation of ( ) and ( )-metyrapol (Figure 11.4).
The assay was validated for metabolic studies which indicated that the enzymatic
reduction of myterapone is enantiospecific. Some authors have circumvented the
poor performance (e.g. broad asymmetric peaks) of particular chiral columns by
reversing the order of columns, i.e. carrying out a chiral–achiral coupling and
exploiting peak compression effects. After chiral resolution in broad zones, the enan-
tiomers are reconcentrated into sharply defined bands using a separate hydrophobic
column for each enantiomer, thereby substantially improving the overall sensitivity
and selectivity. This concept has been applied for the enantioseparation of sub-
stances such as leucovorine (53), manidipine (45), bupivacaine, metoprolol,
oxazepam and terbutaline (54) in plasma.
LC–LC of endogenous compounds in biological fluids has also been reported
(see Table 11.1) and does not differ essentially from the bioanalysis of drugs by
coupled-column LC. For example, Tagesson et al. (59) determined the DNA adduct
8-hydroxydeoxyguanosine in human urine by on-line injecting a sample fraction
eluting from a first polymeric RPLC column into a second ODS column which was
connected to an electrochemical detector. This system was used for assaying in vivo
oxidative DNA damage in cancer patients. High levels of the urinary adduct were
found in patients subjected to body irradiation and chemotherapy.