Page 406 - Multidimensional Chromatography
P. 406
398 Multidimensional Chromatography
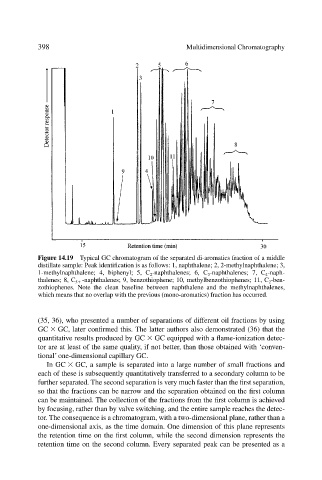
Figure 14.19 Typical GC chromatogram of the separated di-aromatics fraction of a middle
distillate sample: Peak identification is as follows: 1, naphthalene; 2, 2-methylnaphthalene; 3,
1-methylnaphthalene; 4, biphenyl; 5, C 2 -naphthalenes; 6, C 3 -naphthalenes; 7, C 4 -naph-
thalenes; 8, C 5 -naphthalenes; 9, benzothiophene; 10, methylbenzothiophenes; 11, C 2 -ben-
zothiophenes. Note the clean baseline between naphthalene and the methylnaphthalenes,
which means that no overlap with the previous (mono-aromatics) fraction has occurred.
(35, 36), who presented a number of separations of different oil fractions by using
GC
GC, later confirmed this. The latter authors also demonstrated (36) that the
quantitative results produced by GC
GC equipped with a flame-ionization detec-
tor are at least of the same quality, if not better, than those obtained with ‘conven-
tional’ one-dimensional capillary GC.
In GC
GC, a sample is separated into a large number of small fractions and
each of these is subsequently quantitatively transferred to a secondary column to be
further separated. The second separation is very much faster than the first separation,
so that the fractions can be narrow and the separation obtained on the first column
can be maintained. The collection of the fractions from the first column is achieved
by focusing, rather than by valve switching, and the entire sample reaches the detec-
tor. The consequence is a chromatogram, with a two-dimensional plane, rather than a
one-dimensional axis, as the time domain. One dimension of this plane represents
the retention time on the first column, while the second dimension represents the
retention time on the second column. Every separated peak can be presented as a