Page 425 - Multidimensional Chromatography
P. 425
Forensic and Toxicological Applications 417
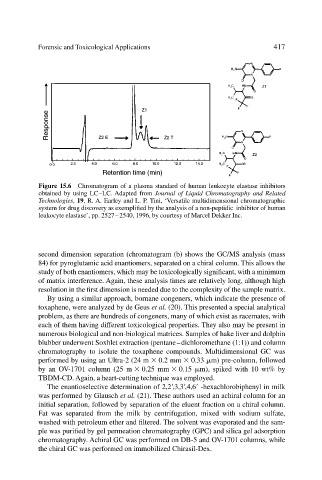
Figure 15.6 Chromatogram of a plasma standard of human leukocyte elastase inhibitors
obtained by using LC–LC. Adapted from Journal of Liquid Chromatography and Related
Technologies, 19, R. A. Earley and L. P. Tini, ‘Versatile multidimensional chromatographic
system for drug discovery as exemplified by the analysis of a non-peptidic inhibitor of human
leukocyte elastase’, pp. 2527–2540, 1996, by courtesy of Marcel Dekker Inc.
second dimension separation (chromatogram (b) shows the GC/MS analysis (mass
84) for pyroglutamic acid enantiomers, separated on a chiral column. This allows the
study of both enantiomers, which may be toxicologically significant, with a minimum
of matrix interference. Again, these analysis times are relatively long, although high
resolution in the first dimension is needed due to the complexity of the sample matrix.
By using a similar approach, bornane congeners, which indicate the presence of
toxaphene, were analyzed by de Geus et al. (20). This presented a special analytical
problem, as there are hundreds of congeners, many of which exist as racemates, with
each of them having different toxicological properties. They also may be present in
numerous biological and non-biological matrices. Samples of hake liver and dolphin
blubber underwent Soxhlet extraction (pentane–dichloromethane (1:1)) and column
chromatography to isolate the toxaphene compounds. Multidimensional GC was
performed by using an Ultra-2 (24 m 0.2 mm 0.33 m) pre-column, followed
by an OV-1701 column (25 m 0.25 mm 0.15 m), spiked with 10 wt% by
TBDM-CD. Again, a heart-cutting technique was employed.
The enantioselective determination of 2,2 ,3,3 ,4,6 -hexachlorobiphenyl in milk
was performed by Glausch et al. (21). These authors used an achiral column for an
initial separation, followed by separation of the eluent fraction on a chiral column.
Fat was separated from the milk by centrifugation, mixed with sodium sulfate,
washed with petroleum ether and filtered. The solvent was evaporated and the sam-
ple was purified by gel permeation chromatography (GPC) and silica gel adsorption
chromatography. Achiral GC was performed on DB-5 and OV-1701 columns, while
the chiral GC was performed on immobilized Chirasil-Dex.