Page 133 - Multifunctional Photocatalytic Materials for Energy
P. 133
Carbon nitride photocatalysts 119
could be reduced to its original state via a sacrificial agent for the next cycle. Fig. 6.5D
shows that the introduction of a sensitizer can efficiently prohibit the charge recombi-
nation. Based on this mechanism, Eosin Y was selected as the sensitizer by Min et al.
[81]. They found that mesoporous carbon nitride sensitized by Eosin Y significantly
extended the absorption threshold of the visible light spectrum to 600 nm. Therefore
the improved light response ability led to a striking apparent quantum efficiency of
19.4% under 550 nm wavelength.
Dye-sensitized carbon nitride is a promising candidate for producing photosynthe-
sis hydrogen energy because it significantly improves the hydrogen production rate.
However, only a limited number of appropriate dyes have been discovered, which has
hindered further improvement in this field. Therefore development and utilization of a
series of robust sensitizers with proper band structures is indeed needed.
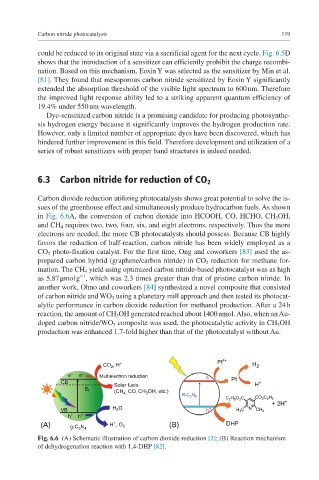
6.3 Carbon nitride for reduction of CO 2
Carbon dioxide reduction utilizing photocatalysts shows great potential to solve the is-
sues of the greenhouse effect and simultaneously produce hydrocarbon fuels. As shown
in Fig. 6.6A, the conversion of carbon dioxide into HCOOH, CO, HCHO, CH 3 OH,
and CH 4 requires two, two, four, six, and eight electrons, respectively. Thus the more
electrons are needed, the more CB photocatalysts should possess. Because CB highly
favors the reduction of half-reaction, carbon nitride has been widely employed as a
CO 2 photo-fixation catalyst. For the first time, Ong and coworkers [83] used the as-
prepared carbon hybrid (graphene/carbon nitride) in CO 2 reduction for methane for-
mation. The CH 4 yield using optimized carbon nitride-based photocatalyst was as high
−1
as 5.87 μmol g , which was 2.3 times greater than that of pristine carbon nitride. In
another work, Ohno and coworkers [84] synthesized a novel composite that consisted
of carbon nitride and WO 3 using a planetary mill approach and then tested its photocat-
alytic performance in carbon dioxide reduction for methanol production. After a 24 h
reaction, the amount of CH 3 OH generated reached about 1400 nmol. Also, when an Au-
doped carbon nitride/WO 3 composite was used, the photocatalytic activity in CH 3 OH
production was enhanced 1.7-fold higher than that of the photocatalyst without Au.
CO 2 , H + Pt 4+ H 2
e – e – Multielectron reduction e
CB Solar fuels Pt H +
(CH 4, CO, CH 3 OH, etc.)
E f
R-C 3 N 4
C 2 H 5 O 2 C CO 2 C 2 H 5
+ 2H +
VB H 2 O h + H 3 C N CH 3
h + h + e
(A) g-C 3 N 4 H , O 2 (B) DHP
+
Fig. 6.6 (A) Schematic illustration of carbon dioxide reduction [2]; (B) Reaction mechanism
of dehydrogenation reaction with 1,4-DHP [82].