Page 219 - Multifunctional Photocatalytic Materials for Energy
P. 219
Metal-based semiconductor nanomaterials for photocatalysis 203
processes and sintering damage, which reduces the surface area and could also cause
a phase transition of the material (i.e., anatase to rutile).
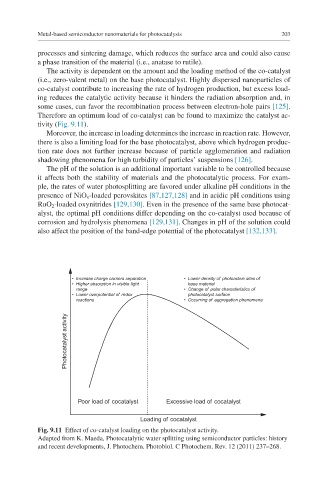
The activity is dependent on the amount and the loading method of the co-catalyst
(i.e., zero-valent metal) on the base photocatalyst. Highly dispersed nanoparticles of
co-catalyst contribute to increasing the rate of hydrogen production, but excess load-
ing reduces the catalytic activity because it hinders the radiation absorption and, in
some cases, can favor the recombination process between electron-hole pairs [125].
Therefore an optimum load of co-catalyst can be found to maximize the catalyst ac-
tivity (Fig. 9.11).
Moreover, the increase in loading determines the increase in reaction rate. However,
there is also a limiting load for the base photocatalyst, above which hydrogen produc-
tion rate does not further increase because of particle agglomeration and radiation
shadowing phenomena for high turbidity of particles’ suspensions [126].
The pH of the solution is an additional important variable to be controlled because
it affects both the stability of materials and the photocatalytic process. For exam-
ple, the rates of water photosplitting are favored under alkaline pH conditions in the
presence of NiO x -loaded perovskites [87,127,128] and in acidic pH conditions using
RuO 2 -loaded oxynitrides [129,130]. Even in the presence of the same base photocat-
alyst, the optimal pH conditions differ depending on the co-catalyst used because of
corrosion and hydrolysis phenomena [129,131]. Changes in pH of the solution could
also affect the position of the band-edge potential of the photocatalyst [132,133].
• Increase charge carriers separation • Lower density of photoactive sites of
• Higher absorption in visible light base material
range • Change of polar characteristics of
• Lower overpotential of redox photocatalyst surface
reactions • Occurring of aggregation phenomena
Photocatalyst activity
Poor load of cocatalyst Excessive load of cocatalyst
Loading of cocatalyst
Fig. 9.11 Effect of co-catalyst loading on the photocatalyst activity.
Adapted from K. Maeda, Photocatalytic water splitting using semiconductor particles: history
and recent developments, J. Photochem. Photobiol. C Photochem. Rev. 12 (2011) 237–268.