Page 240 - Multifunctional Photocatalytic Materials for Energy
P. 240
Photocatalysts for hydrogen generation and organic contaminants degradation 223
A scavenger species preferentially removes holes (or electrons) and thereby traps
electrons (or holes) for a longer duration. The first generation of photocatalytic mate-
rials is considered to be that of metal oxides. TiO 2 was one of the most studied metal
oxides for odor containment, antibacterial and antimicrobial activities, photosplit-
ting of water, generation of H 2 gas in the process, and so on [13]. Along with TiO 2 ,
other metal oxides used for photocatalytic properties are depicted in Fig. 10.4B. The
second-generation photocatalytic materials is that of anion-doped (C, S, N) metal ox-
ides [15]. These doped metal oxides display better photocatalytic activities, which is
attributed to better overlap of the states between 2p orbitals of oxygen and the states
caused by doping. The third-generation photocatalysts comprise nanocomposites of
semiconducting materials and two low band gap photo-responsive materials [16].
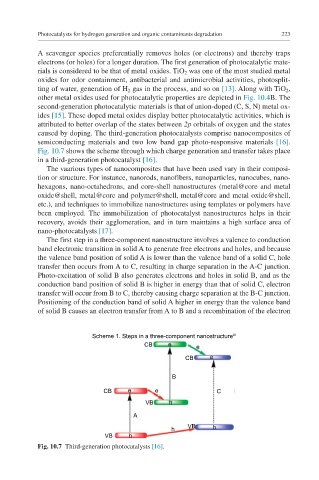
Fig. 10.7 shows the scheme through which charge generation and transfer takes place
in a third-generation photocatalyst [16].
The vaarious types of nanocomposites that have been used vary in their composi-
tion or structure. For instance, nanorods, nanofibers, nanoparticles, nanocubes, nano-
hexagons, nano-octahedrons, and core-shell nanostructures (metal@core and metal
oxide@shell, metal@core and polymer@shell, metal@core and metal oxide@shell,
etc.), and techniques to immobilize nanostructures using templates or polymers have
been employed. The immobilization of photocatalyst nanostructures helps in their
recovery, avoids their agglomeration, and in turn maintains a high surface area of
nano-photocatalysts [17].
The first step in a three-component nanostructure involves a valence to conduction
band electronic transition in solid A to generate free electrons and holes, and because
the valence band position of solid A is lower than the valence band of a solid C, hole
transfer then occurs from A to C, resulting in charge separation in the A-C junction.
Photo-excitation of solid B also generates electrons and holes in solid B, and as the
conduction band position of solid B is higher in energy than that of solid C, electron
transfer will occur from B to C, thereby causing charge separation at the B-C junction.
Positioning of the conduction band of solid A higher in energy than the valence band
of solid B causes an electron transfer from A to B and a recombination of the electron
Scheme 1. Steps in a three-component nanostructure α
CB e e
CB e
B
CB e e C
VB h
A
h VB h
VB h
Fig. 10.7 Third-generation photocatalysts [16].