Page 242 - Multifunctional Photocatalytic Materials for Energy
P. 242
Photocatalysts for hydrogen generation and organic contaminants degradation 225
Potential
(V vs.NHE)
pH = 0
H -evolution cocatalyst
2
(ii)
e –
+
(H /H 2 ) 0 (iii) H 2 e – CB
H +
(i)
H 2 O
(O 2 /H 2 O) +1.23 VB h + O 2 (iii)
h +
(ii)
Semiconductor photocatalyst O 2 -evolution cocatalyst
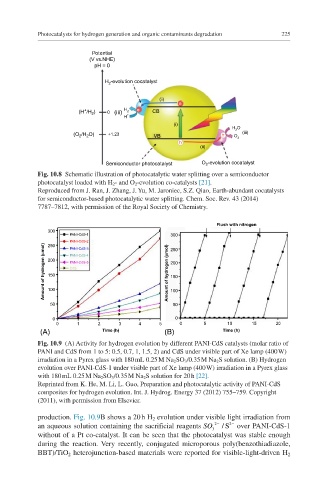
Fig. 10.8 Schematic illustration of photocatalytic water splitting over a semiconductor
photocatalyst loaded with H 2 - and O 2 -evolution co-catalysts [21].
Reproduced from J. Ran, J. Zhang, J. Yu, M. Jaroniec, S.Z. Qiao, Earth-abundant cocatalysts
for semiconductor-based photocatalytic water splitting. Chem. Soc. Rev. 43 (2014)
7787–7812, with permission of the Royal Society of Chemistry.
Flush with nitrogen
300
PANI-CdS-1 300
PANI-CdS-2 250
Amount of hydrogen (µmol) 200 PANI-CdS-5 Amount of hydrogen (µmol) 200
250
PANI-CdS-3
PANI-CdS-4
CdS
150
150
100
50 100
50
0 0
0 1 2 3 4 5 0 5 10 15 20
(A) Time (h) (B) Time (h)
Fig. 10.9 (A) Activity for hydrogen evolution by different PANI-CdS catalysts (molar ratio of
PANI and CdS from 1 to 5: 0.5, 0.7, 1, 1.5, 2) and CdS under visible part of Xe lamp (400 W)
irradiation in a Pyrex glass with 180 mL 0.25 M Na 2 SO 3 /0.35 M Na 2 S solution. (B) Hydrogen
evolution over PANI-CdS-1 under visible part of Xe lamp (400 W) irradiation in a Pyrex glass
with 180 mL 0.25 M Na 2 SO 3 /0.35 M Na 2 S solution for 20 h [22].
Reprinted from K. He, M. Li, L. Guo, Preparation and photocatalytic activity of PANI-CdS
composites for hydrogen evolution. Int. J. Hydrog. Energy 37 (2012) 755–759. Copyright
(2011), with permission from Elsevier.
production. Fig. 10.9B shows a 20 h H 2 evolution under visible light irradiation from
2-
an aqueous solution containing the sacrificial reagents SO 3 2- /S over PANI-CdS-1
without of a Pt co-catalyst. It can be seen that the photocatalyst was stable enough
during the reaction. Very recently, conjugated microporous poly(benzothiadiazole,
BBT)/TiO 2 heterojunction-based materials were reported for visible-light-driven H 2