Page 246 - Multifunctional Photocatalytic Materials for Energy
P. 246
Photocatalysts for hydrogen generation and organic contaminants degradation 229
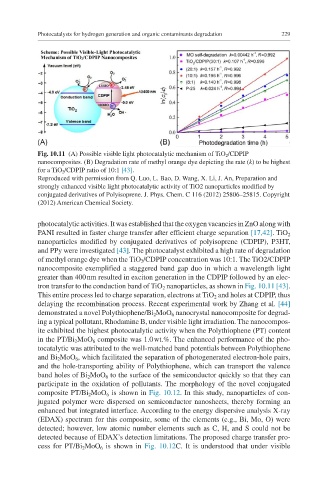
Fig. 10.11 (A) Possible visible light photocatalytic mechanism of TiO 2 /CDPIP
nanocomposites. (B) Degradation rate of methyl orange dye depicting the rate (k) to be highest
for a TiO 2 /CDPIP ratio of 10:1 [43].
Reproduced with permission from Q. Luo, L. Bao, D. Wang, X. Li, J. An, Preparation and
strongly enhanced visible light photocatalytic activity of TiO2 nanoparticles modified by
conjugated derivatives of Polyisoprene. J. Phys. Chem. C 116 (2012) 25806–25815. Copyright
(2012) American Chemical Society.
photocatalytic activities. It was established that the oxygen vacancies in ZnO along with
PANI resulted in faster charge transfer after efficient charge separation [17,42]. TiO 2
nanoparticles modified by conjugated derivatives of polyisoprene (CDPIP), P3HT,
and PPy were investigated [43]. The photocatalyst exhibited a high rate of degradation
of methyl orange dye when the TiO 2 /CDPIP concentration was 10:1. The TiO2/CDPIP
nanocomposite exemplified a staggered band gap duo in which a wavelength light
greater than 400 nm resulted in exciton generation in the CDPIP followed by an elec-
tron transfer to the conduction band of TiO 2 nanoparticles, as shown in Fig. 10.11 [43].
This entire process led to charge separation, electrons at TiO 2 and holes at CDPIP, thus
delaying the recombination process. Recent experimental work by Zhang et al. [44]
demonstrated a novel Polythiophene/Bi 2 MoO 6 nanocrystal nanocomposite for degrad-
ing a typical pollutant, Rhodamine B, under visible light irradiation. The nanocompos-
ite exhibited the highest photocatalytic activity when the Polythiophene (PT) content
in the PT/Bi 2 MoO 6 composite was 1.0 wt.%. The enhanced performance of the pho-
tocatalytic was attributed to the well-matched band potentials between Polythiophene
and Bi 2 MoO 6 , which facilitated the separation of photogenerated electron-hole pairs,
and the hole-transporting ability of Polythiophene, which can transport the valence
band holes of Bi 2 MoO 6 to the surface of the semiconductor quickly so that they can
participate in the oxidation of pollutants. The morphology of the novel conjugated
composite PT/Bi 2 MoO 6 is shown in Fig. 10.12. In this study, nanoparticles of con-
jugated polymer were dispersed on semiconductor nanosheets, thereby forming an
enhanced but integrated interface. According to the energy dispersive analysis X-ray
(EDAX) spectrum for this composite, some of the elements (e.g., Bi, Mo, O) were
detected; however, low atomic number elements such as C, H, and S could not be
detected because of EDAX’s detection limitations. The proposed charge transfer pro-
cess for PT/Bi 2 MoO 6 is shown in Fig. 10.12C. It is understood that under visible