Page 248 - Multifunctional Photocatalytic Materials for Energy
P. 248
Photocatalysts for hydrogen generation and organic contaminants degradation 231
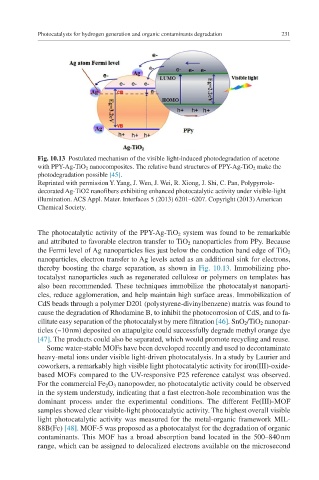
Fig. 10.13 Postulated mechanism of the visible light-induced photodegradation of acetone
with PPY-Ag-TiO 2 nanocomposites. The relative band structures of PPY-Ag-TiO 2 make the
photodegradation possible [45].
Reprinted with permission Y. Yang, J. Wen, J. Wei, R. Xiong, J. Shi, C. Pan, Polypyrrole-
decorated Ag-TiO2 nanofibers exhibiting enhanced photocatalytic activity under visible-light
illumination. ACS Appl. Mater. Interfaces 5 (2013) 6201–6207. Copyright (2013) American
Chemical Society.
The photocatalytic activity of the PPY-Ag-TiO 2 system was found to be remarkable
and attributed to favorable electron transfer to TiO 2 nanoparticles from PPy. Because
the Fermi level of Ag nanoparticles lies just below the conduction band edge of TiO 2
nanoparticles, electron transfer to Ag levels acted as an additional sink for electrons,
thereby boosting the charge separation, as shown in Fig. 10.13. Immobilizing pho-
tocatalyst nanoparticles such as regenerated cellulose or polymers on templates has
also been recommended. These techniques immobilize the photocatalyst nanoparti-
cles, reduce agglomeration, and help maintain high surface areas. Immobilization of
CdS beads through a polymer D201 (polystyrene-divinylbenzene) matrix was found to
cause the degradation of Rhodamine B, to inhibit the photocorrosion of CdS, and to fa-
cilitate easy separation of the photocatalyst by mere filtration [46]. SnO 2 /TiO 2 nanopar-
ticles (~10 nm) deposited on attapulgite could successfully degrade methyl orange dye
[47]. The products could also be separated, which would promote recycling and reuse.
Some water-stable MOFs have been developed recently and used to decontaminate
heavy-metal ions under visible light-driven photocatalysis. In a study by Laurier and
coworkers, a remarkably high visible light photocatalytic activity for iron(III)-oxide-
based MOFs compared to the UV-responsive P25 reference catalyst was observed.
For the commercial Fe 2 O 3 nanopowder, no photocatalytic activity could be observed
in the system understudy, indicating that a fast electron-hole recombination was the
dominant process under the experimental conditions. The different Fe(III)-MOF
samples showed clear visible-light photocatalytic activity. The highest overall visible
light photocatalytic activity was measured for the metal-organic framework MIL-
88B(Fe) [48]. MOF-5 was proposed as a photocatalyst for the degradation of organic
contaminants. This MOF has a broad absorption band located in the 500–840 nm
range, which can be assigned to delocalized electrons available on the microsecond