Page 245 - Multifunctional Photocatalytic Materials for Energy
P. 245
228 Multifunctional Photocatalytic Materials for Energy
1.0
1.0
1st run 2nd run 3rd run 4th run 5th run
0.8 0.8
0.6
C/C 0 0.4 blank C/C 0 0.6
P/T-100°C 0.4
0.2 TiO 2x N x
TiO 2 -200°C 0.2
0.0 P/T-150°C
P/T-200°C 0.0
0 1 2 3 4 5 6 0 6 12 18 24 30
(A) Illumination time (h) (B) Illumination time (h)
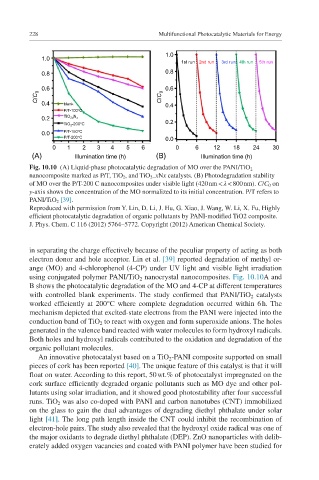
Fig. 10.10 (A) Liquid-phase photocatalytic degradation of MO over the PANI/TiO 2
nanocomposite marked as P/T, TiO 2 , and TiO 2− xNx catalysts. (B) Photodegradation stability
of MO over the P/T-200 C nanocomposites under visible light (420 nm < λ < 800 nm). C/C 0 on
y-axis shows the concentration of the MO normalized to its initial concentration. P/T refers to
PANI/TiO 2 [39].
Reproduced with permission from Y. Lin, D. Li, J. Hu, G. Xiao, J. Wang, W. Li, X. Fu, Highly
efficient photocatalytic degradation of organic pollutants by PANI-modified TiO2 composite.
J. Phys. Chem. C 116 (2012) 5764–5772. Copyright (2012) American Chemical Society.
in separating the charge effectively because of the peculiar property of acting as both
electron donor and hole acceptor. Lin et al. [39] reported degradation of methyl or-
ange (MO) and 4-chlorophenol (4-CP) under UV light and visible light irradiation
using conjugated polymer PANI/TiO 2 nanocrystal nanocomposites. Fig. 10.10A and
B shows the photocatalytic degradation of the MO and 4-CP at different temperatures
with controlled blank experiments. The study confirmed that PANI/TiO 2 catalysts
worked efficiently at 200°C where complete degradation occurred within 6 h. The
mechanism depicted that excited-state electrons from the PANI were injected into the
conduction band of TiO 2 to react with oxygen and form superoxide anions. The holes
generated in the valence band reacted with water molecules to form hydroxyl radicals.
Both holes and hydroxyl radicals contributed to the oxidation and degradation of the
organic pollutant molecules.
An innovative photocatalyst based on a TiO 2 -PANI composite supported on small
pieces of cork has been reported [40]. The unique feature of this catalyst is that it will
float on water. According to this report, 50 wt.% of photocatalyst impregnated on the
cork surface efficiently degraded organic pollutants such as MO dye and other pol-
lutants using solar irradiation, and it showed good photostability after four successful
runs. TiO 2 was also co-doped with PANI and carbon nanotubes (CNT) immobilized
on the glass to gain the dual advantages of degrading diethyl phthalate under solar
light [41]. The long path length inside the CNT could inhibit the recombination of
electron-hole pairs. The study also revealed that the hydroxyl oxide radical was one of
the major oxidants to degrade diethyl phthalate (DEP). ZnO nanoparticles with delib-
erately added oxygen vacancies and coated with PANI polymer have been studied for