Page 31 - Multifunctional Photocatalytic Materials for Energy
P. 31
20 Multifunctional Photocatalytic Materials for Energy
B
6 )
E E 4 1.23 V
e - e - CB 0 J (mA x cm −2 2 Dark
+ E F 1.0 1.2 1.4 1.6 1.8
H /H 2
Cathode Potential(V)
hn E G
O 2 /H 2 O
VB
Anode e -
h +
Light
e -
A e -
e - H 2 O
e - O 2 evolution
H + + e -
H e -
H evolution e - O 2
2
e -
e
H 2 -
e -
Cathode Anode
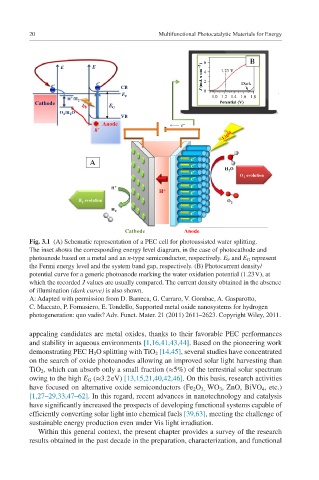
Fig. 3.1 (A) Schematic representation of a PEC cell for photoassisted water splitting.
The inset shows the corresponding energy level diagram, in the case of photocathode and
photoanode based on a metal and an n-type semiconductor, respectively. E F and E G represent
the Fermi energy level and the system band gap, respectively. (B) Photocurrent density/
potential curve for a generic photoanode marking the water oxidation potential (1.23 V), at
which the recorded J values are usually compared. The current density obtained in the absence
of illumination (dark curve) is also shown.
A: Adapted with permission from D. Barreca, G. Carraro, V. Gombac, A. Gasparotto,
C. Maccato, P. Fornasiero, E. Tondello, Supported metal oxide nanosystems for hydrogen
photogeneration: quo vadis? Adv. Funct. Mater. 21 (2011) 2611–2623. Copyright Wiley, 2011.
appealing candidates are metal oxides, thanks to their favorable PEC performances
and stability in aqueous environments [1,16,41,43,44]. Based on the pioneering work
demonstrating PEC H 2 O splitting with TiO 2 [14,45], several studies have concentrated
on the search of oxide photoanodes allowing an improved solar light harvesting than
TiO 2 , which can absorb only a small fraction (≈5%) of the terrestrial solar spectrum
owing to the high E G (≈3.2 eV) [13,15,21,40,42,46]. On this basis, research activities
have focused on alternative oxide semiconductors (Fe 2 O 3, WO 3 , ZnO, BiVO 4 , etc.)
[1,27–29,33,47–62]. In this regard, recent advances in nanotechnology and catalysis
have significantly increased the prospects of developing functional systems capable of
efficiently converting solar light into chemical fuels [39,63], meeting the challenge of
sustainable energy production even under Vis light irradiation.
Within this general context, the present chapter provides a survey of the research
results obtained in the past decade in the preparation, characterization, and functional