Page 33 - Multifunctional Photocatalytic Materials for Energy
P. 33
22 Multifunctional Photocatalytic Materials for Energy
−1
free energy required for the overall process (ΔG° = 237 kJ × mol ) [32,33,38,42]. The
main challenge lies in the complexity of the OER (1), which is both kinetically and
thermodynamically demanding, requiring an overall four-electron/four-proton process
for the evolution of one O 2 molecule [1,3,11,18,20,27,40]. Consequently, the design
of suitable photoanode materials capable of efficiently driving this reaction is an im-
portant hurdle to overcome [65]. Nonetheless, the target semiconductor must possess
an adequate electronic band structure, where the potential of the CB edge should be
more negative than that of the H 2 evolution reaction, and the potential of the VB edge
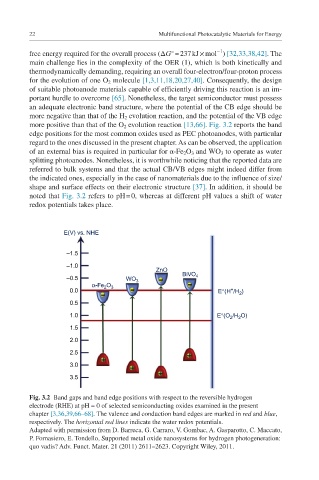
more positive than that of the O 2 evolution reaction [13,66]. Fig. 3.2 reports the band
edge positions for the most common oxides used as PEC photoanodes, with particular
regard to the ones discussed in the present chapter. As can be observed, the application
of an external bias is required in particular for α-Fe 2 O 3 and WO 3 to operate as water
splitting photoanodes. Nonetheless, it is worthwhile noticing that the reported data are
referred to bulk systems and that the actual CB/VB edges might indeed differ from
the indicated ones, especially in the case of nanomaterials due to the influence of size/
shape and surface effects on their electronic structure [37]. In addition, it should be
noted that Fig. 3.2 refers to pH = 0, whereas at different pH values a shift of water
redox potentials takes place.
E(V) vs. NHE
–1.5
–1.0
ZnO
–0.5 WO 3 BiVO 4
α-Fe O 3
2
+
0.0 E°(H /H )
2
0.5
1.0 E°(O /H O)
2
2
1.5
2.0
2.5
3.0
3.5
Fig. 3.2 Band gaps and band edge positions with respect to the reversible hydrogen
electrode (RHE) at pH = 0 of selected semiconducting oxides examined in the present
chapter [3,36,39,66–68]. The valence and conduction band edges are marked in red and blue,
respectively. The horizontal red lines indicate the water redox potentials.
Adapted with permission from D. Barreca, G. Carraro, V. Gombac, A. Gasparotto, C. Maccato,
P. Fornasiero, E. Tondello, Supported metal oxide nanosystems for hydrogen photogeneration:
quo vadis? Adv. Funct. Mater. 21 (2011) 2611–2623. Copyright Wiley, 2011.