Page 37 - Multifunctional Photocatalytic Materials for Energy
P. 37
26 Multifunctional Photocatalytic Materials for Energy
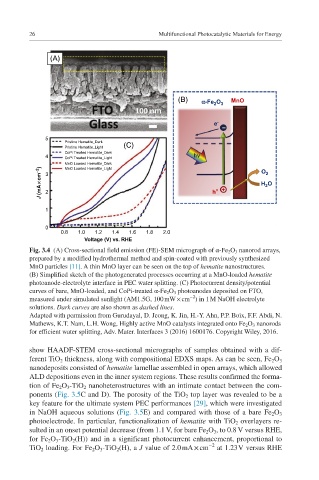
(A)
(B) a-Fe 2 O 3 MnO
e -
–
5
Pristine Hematite_Dark (C)
Pristine Hematite_Light
CoPi Treated Hematite_Dark
4 CoPi Treated Hematite_Light hn
MnO Loaded Hematite_Dark O 2
J (mA ¥cm –2 ) 3 2 h + + H 2 O
MnO Loaded Hematite_Light
1
0
0.8 1.0 1.2 1.4 1.6 1.8 2.0
Voltage (V) vs. RHE
Fig. 3.4 (A) Cross-sectional field emission (FE)-SEM micrograph of α-Fe 2 O 3 nanorod arrays,
prepared by a modified hydrothermal method and spin-coated with previously synthesized
MnO particles [11]. A thin MnO layer can be seen on the top of hematite nanostructures.
(B) Simplified sketch of the photogenerated processes occurring at a MnO-loaded hematite
photoanode-electrolyte interface in PEC water splitting. (C) Photocurrent density/potential
curves of bare, MnO-loaded, and CoPi-treated α-Fe 2 O 3 photoanodes deposited on FTO,
−2
measured under simulated sunlight (AM1.5G, 100 mW × cm ) in 1 M NaOH electrolyte
solutions. Dark curves are also shown as dashed lines.
Adapted with permission from Gurudayal, D. Jeong, K. Jin, H.-Y. Ahn, P.P. Boix, F.F. Abdi, N.
Mathews, K.T. Nam, L.H. Wong, Highly active MnO catalysts integrated onto Fe 2 O 3 nanorods
for efficient water splitting, Adv. Mater. Interfaces 3 (2016) 1600176. Copyright Wiley, 2016.
show HAADF-STEM cross-sectional micrographs of samples obtained with a dif-
ferent TiO 2 thickness, along with compositional EDXS maps. As can be seen, Fe 2 O 3
nanodeposits consisted of hematite lamellae assembled in open arrays, which allowed
ALD depositions even in the inner system regions. These results confirmed the forma-
tion of Fe 2 O 3 -TiO 2 nanoheterostructures with an intimate contact between the com-
ponents (Fig. 3.5C and D). The porosity of the TiO 2 top layer was revealed to be a
key feature for the ultimate system PEC performances [29], which were investigated
in NaOH aqueous solutions (Fig. 3.5E) and compared with those of a bare Fe 2 O 3
photoelectrode. In particular, functionalization of hematite with TiO 2 overlayers re-
sulted in an onset potential decrease (from 1.1 V, for bare Fe 2 O 3 , to 0.8 V versus RHE,
for Fe 2 O 3 -TiO 2 (H)) and in a significant photocurrent enhancement, proportional to
−2
TiO 2 loading. For Fe 2 O 3 -TiO 2 (H), a J value of 2.0 mA × cm at 1.23 V versus RHE