Page 38 - Multifunctional Photocatalytic Materials for Energy
P. 38
Metal oxide electrodes for photo-activated water splitting 27
(A) (B)
(C) (D)
12 1.0
(E) Fe O 3 (F)
2
10 Fe O TiO (H)
0.8 2 3_ 2
J(mA ×cm –2 ) 6 4 Fe O 3 3_ 3_ 2 2 1.8 V J(mA×cm –2 ) 0.6 1.23 V
8
2
Fe O TiO (L)
0.4
2
Fe O TiO (H)
2
2 0.2
1.23 V
0 0.0
0.8 1.0 1.2 1.4 1.6 1.8 0.6 0.8 1.0 1.2 1.4 1.6
Voltage (V) vs. RHE Voltage (V) vs. RHE
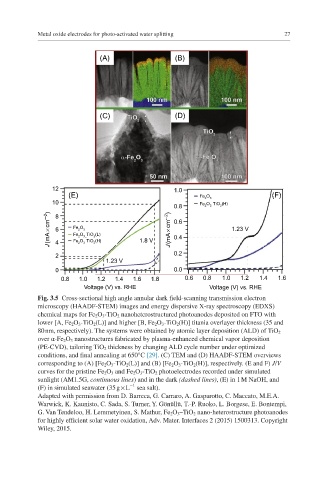
Fig. 3.5 Cross-sectional high angle annular dark field-scanning transmission electron
microscopy (HAADF-STEM) images and energy dispersive X-ray spectroscopy (EDXS)
chemical maps for Fe 2 O 3 -TiO 2 nanoheterostructured photoanodes deposited on FTO with
lower [A, Fe 2 O 3 -TiO 2 (L)] and higher [B, Fe 2 O 3 -TiO 2 (H)] titania overlayer thickness (35 and
80 nm, respectively). The systems were obtained by atomic layer deposition (ALD) of TiO 2
over α-Fe 2 O 3 nanostructures fabricated by plasma-enhanced chemical vapor deposition
(PE-CVD), tailoring TiO 2 thickness by changing ALD cycle number under optimized
conditions, and final annealing at 650°C [29]. (C) TEM and (D) HAADF-STEM overviews
corresponding to (A) [Fe 2 O 3 -TiO 2 (L)] and (B) [Fe 2 O 3 -TiO 2 (H)], respectively. (E and F) J/V
curves for the pristine Fe 2 O 3 and Fe 2 O 3 -TiO 2 photoelectrodes recorded under simulated
sunlight (AM1.5G, continuous lines) and in the dark (dashed lines), (E) in 1 M NaOH, and
−1
(F) in simulated seawater (35 g × L sea salt).
Adapted with permission from D. Barreca, G. Carraro, A. Gasparotto, C. Maccato, M.E.A.
Warwick, K. Kaunisto, C. Sada, S. Turner, Y. Gönüllü, T.-P. Ruoko, L. Borgese, E. Bontempi,
G. Van Tendeloo, H. Lemmetyinen, S. Mathur, Fe 2 O 3 –TiO 2 nano-heterostructure photoanodes
for highly efficient solar water oxidation, Adv. Mater. Interfaces 2 (2015) 1500313. Copyright
Wiley, 2015.