Page 97 - Multifunctional Photocatalytic Materials for Energy
P. 97
86 Multifunctional Photocatalytic Materials for Energy
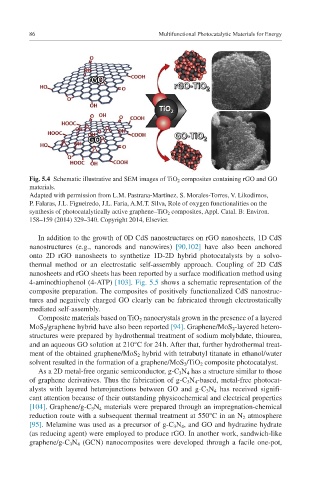
Fig. 5.4 Schematic illustrative and SEM images of TiO 2 composites containing rGO and GO
materials.
Adapted with permission from L.M. Pastrana-Martínez, S. Morales-Torres, V. Likodimos,
P. Falaras, J.L. Figueiredo, J.L. Faria, A.M.T. Silva, Role of oxygen functionalities on the
synthesis of photocatalytically active graphene–TiO 2 composites, Appl. Catal. B: Environ.
158–159 (2014) 329–340. Copyright 2014, Elsevier.
In addition to the growth of 0D CdS nanostructures on rGO nanosheets, 1D CdS
nanostructures (e.g., nanorods and nanowires) [90,102] have also been anchored
onto 2D rGO nanosheets to synthetize 1D-2D hybrid photocatalysts by a solvo-
thermal method or an electrostatic self-assembly approach. Coupling of 2D CdS
nanosheets and rGO sheets has been reported by a surface modification method using
4- aminothiophenol (4-ATP) [103]. Fig. 5.5 shows a schematic representation of the
composite preparation. The composites of positively functionalized CdS nanostruc-
tures and negatively charged GO clearly can be fabricated through electrostatically
mediated self-assembly.
Composite materials based on TiO 2 nanocrystals grown in the presence of a layered
MoS 2 /graphene hybrid have also been reported [94]. Graphene/MoS 2 -layered hetero-
structures were prepared by hydrothermal treatment of sodium molybdate, thiourea,
and an aqueous GO solution at 210°C for 24 h. After that, further hydrothermal treat-
ment of the obtained graphene/MoS 2 hybrid with tetrabutyl titanate in ethanol/water
solvent resulted in the formation of a graphene/MoS 2 /TiO 2 composite photocatalyst.
As a 2D metal-free organic semiconductor, g-C 3 N 4 has a structure similar to those
of graphene derivatives. Thus the fabrication of g-C 3 N 4 -based, metal-free photocat-
alysts with layered heterojunctions between GO and g-C 3 N 4 has received signifi-
cant attention because of their outstanding physicochemical and electrical properties
[104]. Graphene/g-C 3 N 4 materials were prepared through an impregnation-chemical
reduction route with a subsequent thermal treatment at 550°C in an N 2 atmosphere
[95]. Melamine was used as a precursor of g-C 3 N 4 , and GO and hydrazine hydrate
(as reducing agent) were employed to produce rGO. In another work, sandwich-like
graphene/g-C 3 N 4 (GCN) nanocomposites were developed through a facile one-pot,