Page 102 - Multifunctional Photocatalytic Materials for Energy
P. 102
Graphene photocatalysts 91
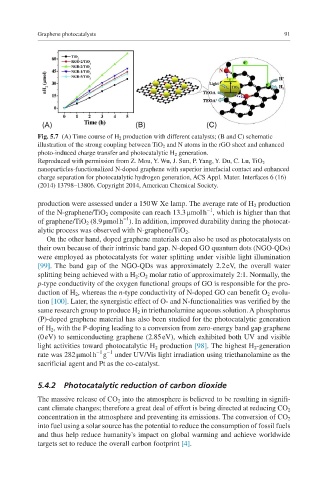
Fig. 5.7 (A) Time course of H 2 production with different catalysts; (B and C) schematic
illustration of the strong coupling between TiO 2 and N atoms in the rGO sheet and enhanced
photo-induced charge transfer and photocatalytic H 2 generation.
Reproduced with permission from Z. Mou, Y. Wu, J. Sun, P. Yang, Y. Du, C. Lu, TiO 2
nanoparticles-functionalized N-doped graphene with superior interfacial contact and enhanced
charge separation for photocatalytic hydrogen generation, ACS Appl. Mater. Interfaces 6 (16)
(2014) 13798–13806. Copyright 2014, American Chemical Society.
production were assessed under a 150 W Xe lamp. The average rate of H 2 production
−1
of the N-graphene/TiO 2 composite can reach 13.3 μmol h , which is higher than that
−1
of graphene/TiO 2 (8.9 μmol h ). In addition, improved durability during the photocat-
alytic process was observed with N-graphene/TiO 2 .
On the other hand, doped graphene materials can also be used as photocatalysts on
their own because of their intrinsic band gap. N-doped GO quantum dots (NGO-QDs)
were employed as photocatalysts for water splitting under visible light illumination
[99]. The band gap of the NGO-QDs was approximately 2.2 eV, the overall water
splitting being achieved with a H 2 :O 2 molar ratio of approximately 2:1. Normally, the
p-type conductivity of the oxygen functional groups of GO is responsible for the pro-
duction of H 2 , whereas the n-type conductivity of N-doped GO can benefit O 2 evolu-
tion [100]. Later, the synergistic effect of O- and N-functionalities was verified by the
same research group to produce H 2 in triethanolamine aqueous solution. A phosphorus
(P)-doped graphene material has also been studied for the photocatalytic generation
of H 2 , with the P-doping leading to a conversion from zero-energy band gap graphene
(0 eV) to semiconducting graphene (2.85 eV), which exhibited both UV and visible
light activities toward photocatalytic H 2 production [98]. The highest H 2 -generation
−1 −1
rate was 282 μmol h g under UV/Vis light irradiation using triethanolamine as the
sacrificial agent and Pt as the co-catalyst.
5.4.2 Photocatalytic reduction of carbon dioxide
The massive release of CO 2 into the atmosphere is believed to be resulting in signifi-
cant climate changes; therefore a great deal of effort is being directed at reducing CO 2
concentration in the atmosphere and preventing its emissions. The conversion of CO 2
into fuel using a solar source has the potential to reduce the consumption of fossil fuels
and thus help reduce humanity's impact on global warming and achieve worldwide
targets set to reduce the overall carbon footprint [4].