Page 104 - Multifunctional Photocatalytic Materials for Energy
P. 104
Graphene photocatalysts 93
tourmaline co-doped TiO 2 nanocomposites (GT/T) [115]. GT/T exhibited significantly
improved activity compared to the graphene-loaded TiO 2 , tourmaline-loaded TiO 2 ,
and bare TiO 2 . This enhancement was attributed to the synergistic effect of graphene
and tourmaline. Specifically, both graphene and tourmaline can improve electron-hole
separation, whereas graphene can reduce the band gap of TiO 2 . As a result, GT/T led
to the enhanced MeOH production rate via photocatalytic CO 2 reduction, which was
21 times higher than that of bare TiO 2 .
A well-defined nanocomposite interface, such as a 2D–2D composite, is import-
ant in preparing highly efficient graphene-based photocatalysts [110]. Robust hollow
spheres consisting of molecular-scale alternating titania (Ti 0.91 O 2 ) nanosheets and
graphene nanosheets were used for the photocatalytic reduction of CO 2 using a 300
W Xe arc lamp [116]. Because both TiO 2 and graphene nanosheets are 2D struc-
tures, graphene and Ti 0.91 O 2 had a close and large contact surface area. The prepared
samples exhibited a high CO formation rate via the photocatalytic CO 2 reduction,
which was nine times higher than that of the commercial P25, because of its fast
electron- hole separation and good light utilization. 2D-2D layered photocatalysts
based on sandwich-like graphene-g-C 3 N 4 (GCN) composite showed enhanced visible
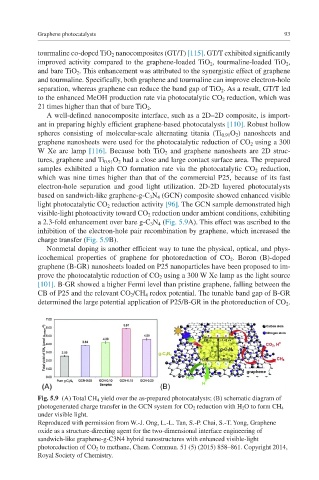
light photocatalytic CO 2 reduction activity [96]. The GCN sample demonstrated high
visible- light photoactivity toward CO 2 reduction under ambient conditions, exhibiting
a 2.3-fold enhancement over bare g-C 3 N 4 (Fig. 5.9A). This effect was ascribed to the
inhibition of the electron-hole pair recombination by graphene, which increased the
charge transfer (Fig. 5.9B).
Nonmetal doping is another efficient way to tune the physical, optical, and phys-
icochemical properties of graphene for photoreduction of CO 2 . Boron (B)-doped
graphene (B-GR) nanosheets loaded on P25 nanoparticles have been proposed to im-
prove the photocatalytic reduction of CO 2 using a 300 W Xe lamp as the light source
[101]. B-GR showed a higher Fermi level than pristine graphene, falling between the
CB of P25 and the relevant CO 2 /CH 4 redox potential. The tunable band gap of B-GR
determined the large potential application of P25/B-GR in the photoreduction of CO 2 .
Fig. 5.9 (A) Total CH 4 yield over the as-prepared photocatalysts; (B) schematic diagram of
photogenerated charge transfer in the GCN system for CO 2 reduction with H 2 O to form CH 4
under visible light.
Reproduced with permission from W.-J. Ong, L.-L. Tan, S.-P. Chai, S.-T. Yong, Graphene
oxide as a structure-directing agent for the two-dimensional interface engineering of
sandwich-like graphene-g-C3N4 hybrid nanostructures with enhanced visible-light
photoreduction of CO 2 to methane, Chem. Commun. 51 (5) (2015) 858–861. Copyright 2014,
Royal Society of Chemistry.