Page 119 - Book Hosokawa Nanoparticle Technology Handbook
P. 119
2.5 PORE STRUCTURE FUNDAMENTALS
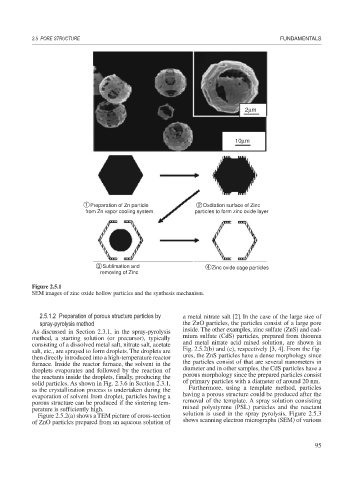
2μm
10μm
1 Preparation of Zn particle 2 Oxdiation surface of Zinc
from Zn vapor cooling system particles to form zinc oxide layer
3 Sublimation and 4 Zinc oxide cage particles
removing of Zinc
Figure 2.5.1
SEM images of zinc oxide hollow particles and the synthesis mechanism.
2.5.1.2 Preparation of porous structure particles by a metal nitrate salt [2]. In the case of the large size of
spray-pyrolysis method the ZnO particles, the particles consist of a large pore
As discussed in Section 2.3.1, in the spray-pyrolysis inside. The other examples, zinc sulfate (ZnS) and cad-
method, a starting solution (or precursor), typically mium sulfate (CdS) particles, prepared from thiourea
consisting of a dissolved metal salt, nitrate salt, acetate and metal nitrate acid mixed solution, are shown in
salt, etc., are sprayed to form droplets. The droplets are Fig. 2.5.2(b) and (c), respectively [3, 4]. From the fig-
then directly introduced into a high-temperature reactor ures, the ZnS particles have a dense morphology since
furnace. Inside the reactor furnace, the solvent in the the particles consist of that are several nanometers in
droplets evaporates and followed by the reaction of diameter and in other samples, the CdS particles have a
the reactants inside the droplets, finally, producing the porous morphology since the prepared particles consist
solid particles. As shown in Fig. 2.3.6 in Section 2.3.1, of primary particles with a diameter of around 20 nm.
as the crystallization process is undertaken during the Furthermore, using a template method, particles
evaporation of solvent from droplet, particles having a having a porous structure could be produced after the
porous structure can be produced if the sintering tem- removal of the template. A spray solution consisting
perature is sufficiently high. mixed polystyrene (PSL) particles and the reactant
Figure 2.5.2(a) shows a TEM picture of cross-section solution is used in the spray pyrolysis. Figure 2.5.3
of ZnO particles prepared from an aqueous solution of shows scanning electron micrographs (SEM) of various
95