Page 114 - Book Hosokawa Nanoparticle Technology Handbook
P. 114
FUNDAMENTALS CH. 2 STRUCTURAL CONTROL OF NANOPARTICLES
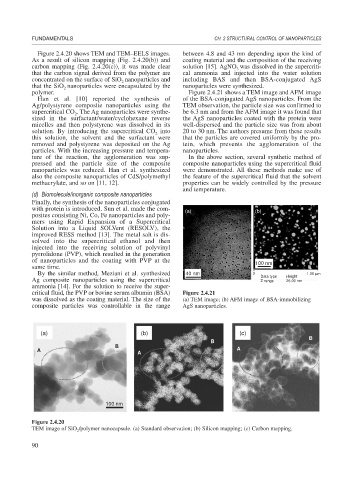
Figure 2.4.20 shows TEM and TEM–EELS images. between 4.8 and 43 nm depending upon the kind of
As a result of silicon mapping (Fig. 2.4.20(b)) and coating material and the composition of the receiving
carbon mapping (Fig. 2.4.20(c)), it was made clear solution [15]. AgNO was dissolved in the supercriti-
3
that the carbon signal derived from the polymer are cal ammonia and injected into the water solution
concentrated on the surface of SiO nanoparticles and including BAS and then BSA-conjugated AgS
2
that the SiO nanoparticles were encapsulated by the nanoparticles were synthesized.
2
polymer. Figure 2.4.21 shows a TEM image and AFM image
Han et al. [10] reported the synthesis of of the BSA-conjugated AgS nanoparticles. From the
Ag/polystyrene composite nanoparticles using the TEM observation, the particle size was confirmed to
supercritical CO . The Ag nanoparticles were synthe- be 6.3 nm and from the AFM image it was found that
2
sized in the surfactant/water/cyclohexane reverse the AgS nanoparticles coated with the protein were
micelles and then polystyrene was dissolved in its well-dispersed and the particle size was from about
solution. By introducing the supercritical CO into 20 to 30 nm. The authors presume from these results
2
this solution, the solvent and the surfactant were that the particles are covered uniformly by the pro-
removed and polystyrene was deposited on the Ag tein, which prevents the agglomeration of the
particles. With the increasing pressure and tempera- nanoparticles.
ture of the reaction, the agglomeration was sup- In the above section, several synthetic method of
pressed and the particle size of the composite composite nanoparticles using the supercritical fluid
nanoparticles was reduced. Han et al. synthesized were demonstrated. All these methods make use of
also the composite nanoparticles of CdS/polymethyl the feature of the supercritical fluid that the solvent
methacrylate, and so on [11, 12]. properties can be widely controlled by the pressure
and temperature.
(d) Biomolecule/inorganic composite nanoparticles
Finally, the synthesis of the nanoparticles conjugated
with protein is introduced. Sun et al. made the com- (a) (b)
posites consisting Ni, Co, Fe nanoparticles and poly-
mers using Rapid Expansion of a Supercritical
Solution into a Liquid SOLVent (RESOLV), the
improved RESS method [13]. The metal salt is dis-
solved into the supercritical ethanol and then
injected into the receiving solution of polyvinyl
pyrrolidone (PVP), which resulted in the generation
of nanoparticles and the coating with PVP at the 100 nm
same time.
By the similar method, Meziani et al. synthesized 40 nm 0 Data type Height 1.00 μm
Ag composite nanoparticles using the supercritical Z range 25.00 nm
ammonia [14]. For the solution to receive the super-
critical fluid, the PVP or bovine serum albumin (BSA) Figure 2.4.21
was dissolved as the coating material. The size of the (a) TEM image; (b) AFM image of BSA-immobilizing
composite particles was controllable in the range AgS nanoparticles.
(a) (b) (c)
B
B
B
A A
A
100 nm
Figure 2.4.20
TEM image of SiO /polymer nanocapsule. (a) Standard observation; (b) Silicon mapping; (c) Carbon mapping.
2
90