Page 113 - Book Hosokawa Nanoparticle Technology Handbook
P. 113
2.4 COMPOSITE STRUCTURE FUNDAMENTALS
Table 2.4.2
Overview of approaches to produce fine particles by the use of supercritical fluid.
a) RESS method Method of producing particles by rapid expansion and reduction of density of
supercritical fluid with dissolved substance to the atmospheric pressure through nozzle
b) PGSS method Using freezing point depression due to the presence of CO , solid material that is
2
normally impossible to be sprayed can be sprayed as a liquid. Method of depositing
solid by cooling effect and decompression of CO caused by rapid expansion
2
c) GAS method Method of depositing crystal by introducing supercritical fluid as a poor solvent into
the solution
d) SAS method Method of causing crystallization by injecting solution into the supercritical fluid as a
poor solvent through nozzle
e) SEDS method Method of depositing particles by raising dispersion efficiency of supercritical fluid
and solvent by mixing at the nozzle tip, introducing solvent, supercritical fluid, and
entrainer through two or three fluid coaxial nozzle
f) Supercritical hydrothermal Nanoparticle synthesis method for metallic oxide made by hydrolysis and dehydration
synthesis method reaction of metal salt solution in supercritical water. By this method, it is possible to
synthesize nanoparticles with high crystallinity and monodispersibility, and size and
shape of particles can be controlled by adjusting temperature and pressure
g) Micro-emulsion method Method of synthesizing particles in the droplet of water in CO -type microemulsion
2
formed in the supercritical CO 2
Pd
CNT Energy (kev)
Pd CNT
Pd
50 nm 100 nm
(a) (b) (c) 50 nm
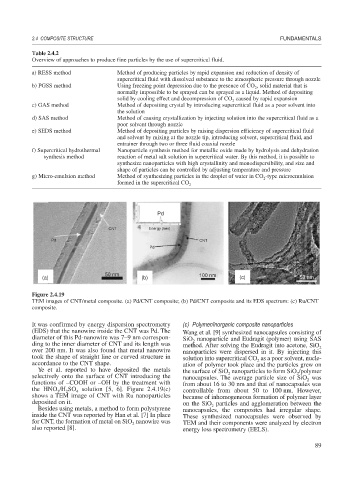
Figure 2.4.19
TEM images of CNT/metal composite. (a) Pd/CNT composite; (b) Pd/CNT composite and its EDS spectrum: (c) Ru/CNT
composite.
It was confirmed by energy dispersion spectrometry (c) Polymer/inorganic composite nanoparticles
(EDS) that the nanowire inside the CNT was Pd. The Wang et al. [9] synthesized nanocapsules consisting of
diameter of this Pd-nanowire was 7–9 nm correspon- SiO nanoparticle and Eudragit (polymer) using SAS
2
ding to the inner diameter of CNT and its length was method. After solving the Eudragit into acetone, SiO 2
over 200 nm. It was also found that metal nanowire nanoparticles were dispersed in it. By injecting this
took the shape of straight line or curved structure in solution into supercritical CO as a poor solvent, nucle-
2
accordance to the CNT shape. ation of polymer took place and the particles grew on
Ye et al. reported to have deposited the metals the surface of SiO nanoparticles to form SiO /polymer
2
2
selectively onto the surface of CNT introducing the nanocapsules. The average particle size of SiO was
2
functions of –COOH or –OH by the treatment with from about 16 to 30 nm and that of nanocapsules was
the HNO /H SO solution [5, 6]. Figure 2.4.19(c) controllable from about 50 to 100 nm. However,
3
2
4
shows a TEM image of CNT with Ru nanoparticles because of inhomogeneous formation of polymer layer
deposited on it. on the SiO particles and agglomeration between the
2
Besides using metals, a method to form polystyrene nanocapsules, the composites had irregular shape.
inside the CNT was reported by Han et al. [7] In place These synthesized nanocapsules were observed by
for CNT, the formation of metal on SiO nanowire was TEM and their components were analyzed by electron
2
also reported [8]. energy loss spectrometry (EELS).
89