Page 141 - Book Hosokawa Nanoparticle Technology Handbook
P. 141
3.1 INTRODUCTION OF NANOPARTICLE DISPERSION AND AGGREGATION BEHAVIOR FUNDAMENTALS
low solid fraction about 20–30 vol%. If solid fraction
is not so high, less than 10%, it is possible to control Free Si-OH (3750 cm )
-1
aggregation and dispersion behavior by using DLVO H-bonded Si-OH (3660 cm )
-1
type interaction, however, with increase of solid frac-
tion in suspension, the additional repulsive interaction
is needed to disperse nanoparticles.
In order to produce the steric repulsive force for the 260 nm
dispersion of particles at relatively high solid fraction
condition, surface modification by the adsorption of
surfactant or reaction with silane coupling agent is
generally used. Special consideration on such tech- Absorbance 87 nm
nique is necessary for the nanoparticles. For example,
the molecular weight of polymer dispersant is gener- 60 nm
ally recommended at about 10,000 g/mol for the dis-
persion of fine powder with submicronmeter in
diameter. Since the size of dispersants with such 30 nm
molecular weight is ranging from several angstroms
to several nanometers in liquid, it is difficult for them
to enter between the particle surfaces as estimated in
Fig. 3.1.2. Furthermore, since such large dispersants 8 nm
have almost equivalent size to the nanoparticles, poly-
mer dispersants sometimes form bridge between par- 4000 3600 3200 2800
-1
ticles, and promote the aggregation of particles and Wavenumbers (cm )
consequently suspension viscosity increases. The
detail examples will be introduced in Section 3.7.2. Figure 3.1.3
Effect of particle diameter on FT-IR spectrum (prepared by
Stöber method).
3.1.4. Surface molecular-level structure of
nanoparticles [3]
between O and H atoms in neighboring Si–OH group
Surface molecular-level structure also changes with increased from 3.0 to 3.17 Å as particle size decreased
decrease of particle size down to nanometer scale. For from several hundreds nanometer to 8 nm. If the dis-
example, Stöber’s process is a very popular method to tance between O and H atoms changed by 0.2 Å, the
prepare uniform spherical alkoxide-derived silica parti- potential energy would have decreased about 20 %, as
cles. The particle diameter of the silica was controlled calculated from an ab initio study [4] of the interaction
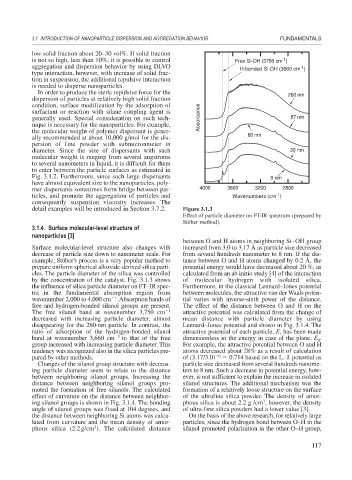
by the concentration of the catalyst. Fig. 3.1.3 shows of molecular hydrogen with isolated silica.
the influence of silica particle diameter on FT–IR spec- Furthermore, in the classical Lennard–Jones potential
tra in the fundamental absorption region from between molecules, the attractive van der Waals poten-
1
wavenumber 2,000 to 4,000 cm . Absorption bands of tial varies with inverse-sixth power of the distance.
free and hydrogen-bonded silanol groups are present. The effect of the distance between O and H on the
The free silanol band at wavenumber 3,750 cm 1 attractive potential was calculated from the change of
decreased with increasing particle diameter, almost mean distance with particle diameter by using
disappearing for the 260-nm particle. In contrast, the Lennard–Jones potential and shown in Fig. 3.1.4. The
ratio of adsorption of the hydrogen-bonded silanol attractive potential of each particle, E, has been made
band at wavenumber 3,660 cm 1 to that of the free dimensionless in the energy in case of the plane, E .
0
group increased with increasing particle diameter. This For example, the attractive potential between O and H
tendency was recognized also in the silica particles pre- atoms decreased about 28% as a result of calculation
pared by other methods. of (3.17/3.0) 6 0.714 based on the L.-J. potential as
Changes of the silanol group structure with decreas- particle size decreased from several hundreds nanome-
ing particle diameter seem to relate to the distance ters to 8 nm. Such a decrease in potential energy, how-
between neighboring silanol groups. Increasing the ever, is not sufficient to explain the increase in isolated
distance between neighboring silanol groups pro- silanol structures. The additional mechanism was the
moted the formation of free silanols. The calculated formation of a relatively loose structure on the surface
effect of curvature on the distance between neighbor- of the ultrafine silica powder. The density of amor-
3
ing silanol groups is shown in Fig. 3.1.4. The bonding phous silica is about 2.2 g /cm , however, the density
angle of silanol groups was fixed at 104 degrees, and of ultra-fine silica powders had a lower value [3].
the distance between neighboring Si atoms was calcu- On the basis of the above research, for relatively large
lated from curvature and the mean density of amor- particles, since the hydrogen bond between O–H in the
3
phous silica (2.2g/cm ). The calculated distance silanol promoted polarization in the other O–H group,
117