Page 142 - Book Hosokawa Nanoparticle Technology Handbook
P. 142
FUNDAMENTALS CH. 3 CHARACTERISTICS AND BEHAVIOR OF NANOPARTICLES AND ITS DISPERSION SYSTEMS
0.34 E/E 0 1.0
Distance between Si-OH (nm) 0.32 silica density = 2.2 g/cm 3 0.6 Atractive energy ratio, E/E 0 ( − )
0.8
0.33
key parameter
0.4
Si-OH distance
0.31
distance
0.0
0.30 OH E/E 0 0.2
10 0 10 1 10 2 10 3 10 4
Particle diameter, d EM (nm)
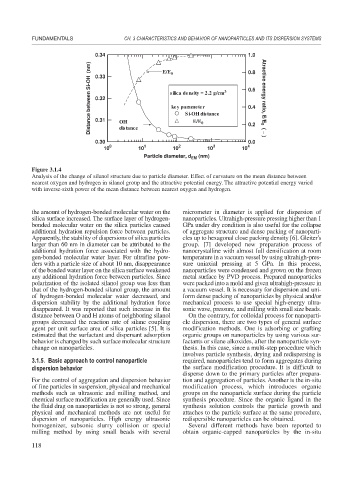
Figure 3.1.4
Analysis of the change of silanol structure due to particle diameter. Effect of curvature on the mean distance between
nearest oxygen and hydrogen in silanol group and the attractive potential energy. The attractive potential energy varied
with inverse-sixth power of the mean distance between nearest oxygen and hydrogen.
the amount of hydrogen-bonded molecular water on the micrometer in diameter is applied for dispersion of
silica surface increased. The surface layer of hydrogen- nanoparticles. Ultrahigh-pressure pressing higher than 1
bonded molecular water on the silica particles caused GPa under dry condition is also useful for the collapse
additional hydration repulsion force between particles. of aggregate structure and dense packing of nanoparti-
Apparently, the stability of dispersions of silica particles cles up to hexagonal close packing density [6]. Gleiter’s
larger than 60 nm in diameter can be attributed to the group. [7] developed new preparation process of
additional hydration force associated with the hydro- nanocrystalline with almost full densification at room
gen-bonded molecular water layer. For ultrafine pow- temperature in a vacuum vessel by using ultrahigh-pres-
ders with a particle size of about 10 nm, disappearance sure uniaxial pressing at 5 GPa. In this process,
of the bonded water layer on the silica surface weakened nanoparticles were condensed and grown on the frozen
any additional hydration force between particles. Since metal surface by PVD process. Prepared nanoparticles
polarization of the isolated silanol group was less than were packed into a mold and given ultrahigh-pressure in
that of the hydrogen-bonded silanol group, the amount a vacuum vessel. It is necessary for dispersion and uni-
of hydrogen-bonded molecular water decreased, and form dense packing of nanoparticles by physical and/or
dispersion stability by the additional hydration force mechanical process to use special high-energy ultra-
disappeared. It was reported that such increase in the sonic wave, pressure, and milling with small size beads.
distance between O and H atoms of neighboring silanol On the contrary, for colloidal process for nanoparti-
groups decreased the reaction rate of silane coupling cle dispersion, there are two types of general surface
agent per unit surface area of silica particles [5]. It is modification methods. One is adsorbing or grafting
estimated that the surfactant and dispersant adsorption organic groups on nanoparticles by using various sur-
behavior is changed by such surface molecular structure factants or silane alkoxides, after the nanoparticle syn-
change on nanoparticles. thesis. In this case, since a multi-step procedure which
involves particle synthesis, drying and redispersing is
3.1.5. Basic approach to control nanoparticle required, nanoparticles tend to form aggregates during
dispersion behavior the surface modification procedure. It is difficult to
disperse down to the primary particles after prepara-
For the control of aggregation and dispersion behavior tion and aggregation of particles. Another is the in-situ
of fine particles in suspension, physical and mechanical modification process, which introduces organic
methods such as ultrasonic and milling method, and groups on the nanoparticle surface during the particle
chemical surface modification are generally used. Since synthesis procedure. Since the organic ligand in the
the fluid drag on nanoparticles is not so strong, general synthesis solution controls the particle growth and
physical and mechanical methods are not useful for attaches to the particle surface at the same procedure,
dispersion of nanoparticles. High energy ultrasonic redispersible nanoparticles can be obtained.
homogenizer, subsonic slurry collision or special Several different methods have been reported to
milling method by using small beads with several obtain organic-capped nanoparticles by the in-situ
118