Page 494 - Book Hosokawa Nanoparticle Technology Handbook
P. 494
APPLICATIONS 11 DEVELOPMENT OF NEW PHOSPHORS
synthesis of 300–400 nm Y O :Eu spherical phosphor
3
2
by uniform coprecipitation method [24]. The lumi-
nance of the nanophosphor under 147 nm excitation is
comparable to that of the conventional sample syn-
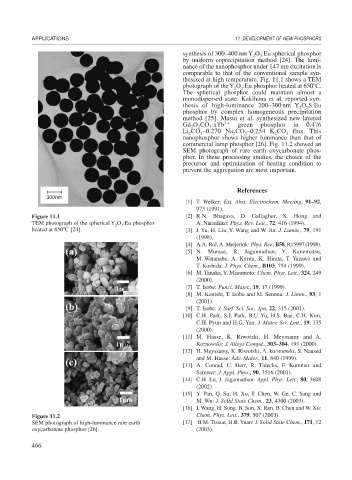
thesized at high temperature. Fig. 11.1 shows a TEM
photograph of the Y O :Eu phosphor heated at 650 C.
3
2
The spherical phosphor could maintain almost a
monodispersed state. Kakihana et al. reported syn-
thesis of high-luminance 200–300 nm Y O S:Eu
2
2
phosphor by complex homogeneous precipitation
method [25]. Masui et al. synthesized new layered
Gd O CO :xTb 3 green phosphor in 0.476
2
3
2
Li CO –0.270 Na CO –0.254 K CO 3 flux. This
2
3
2
3
2
nanophosphor shows higher luminance than that of
commercial lamp phosphor [26]. Fig. 11.2 showed an
SEM photograph of rare earth oxycarbonate phos-
phor. In these processing studies, the choice of the
precursor and optimization of heating condition to
prevent the aggregation are most important.
References
300nm
[1] T. Welker: Ext. Abst. Electrochem. Meeting, 91–92,
973 (1991).
Figure 11.1 [2] R.N. Bhagava, D. Gallagher, X. Hong and
TEM photograph of the spherical Y O :Eu phosphor A. Narmikko: Phys. Rev. Lett., 72, 416 (1994).
3
2
heated at 650 C [24]. [3] J. Yu, H. Liu, Y. Wang and W. Jia: J. Lumin., 79, 191
(1998).
[4] A.A. Bol, A. Meijerink: Phys. Rev., B58, R15997 (1998).
[5] N. Murase, R. Jagannathan, Y. Kanematsu,
M. Watanabe, A. Kurita, K. Hirata, T. Yazawa and
T. Kushida: J. Phys. Chem., B103, 754 (1999).
[6] M. Tanaka, Y. Masumoto: Chem. Phys. Lett., 324, 249
(2000).
[7] T. Isobe: Funct. Mater., 19, 17 (1999).
[8] M. Konishi, T. Isobe and M. Sennna: J. Limin., 93, 1
(2001).
[9] T. Isobe: J. Surf. Sci. Soc. Jpn, 22, 315 (2001).
[10] C.H. Park, S.J. Park, B.U. Yu, H.S. Bae, C.H. Kim,
C.H. Pyun and H.G. Yan: J. Mater. Sci. Lett., 19, 335
(2000).
[11] M. Hasse, K. Riwotzki, H. Meyssamy and A.
Kornowski: J. Alloys Compd., 303–304, 191 (2000).
[12] H. Meyssamy, K. Riwotzki, A. kornnowki, S. Naused
and M. Hasse: Adv. Mater., 11, 840 (1999).
[13] A. Conrad, U. Herr, R. Tidecks, F. Kummer and
Samwer: J. Appl. Phys., 90, 3516 (2001).
[14] C.H. Lu, J. Jagannathan: Appl. Phys. Lett., 80, 3608
(2002).
[15] Y. Pan, Q. Su, H. Xu, T. Chen, W. Ge, C. Yang and
M. Wu: J. Solid State Chem., 23, 4300 (2003).
[16] J. Wang, H. Song, B. Sun, X. Ren, B. Chen and W. Xu:
Figure 11.2 Chem. Phys. Lett., 379, 507 (2003).
SEM photograph of high-luminance rare earth [17] B.M. Tissue, H.B. Yuan: J. Solid State Chem., 171, 12
oxycarbonate phosphor [26]. (2003).
466