Page 601 - Book Hosokawa Nanoparticle Technology Handbook
P. 601
36 DEVELOPMENT OF A HIGH-PERFORMANCE SECONDARY BATTERY APPLICATIONS
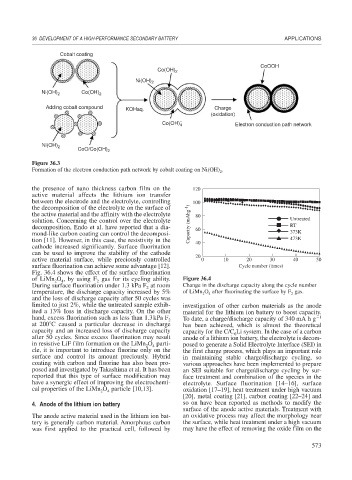
Cobalt coating
CoOOH
Co(OH) 2
Ni(OH) 2
Ni(OH) 2 Co(OH) 2
Adding cobalt compound KOHaq. Charge
(oxidation)
−
Co(OH) 4 Electron conduction path network
Ni(OH) 2
CoO/Co(OH) 2
Figure 36.3
Formation of the electron conduction path network by cobalt coating on Ni(OH) .
2
the presence of nano thickness carbon film on the 120
active material affects the lithium ion transfer
between the electrode and the electrolyte, controlling 100
the decomposition of the electrolyte on the surface of
the active material and the affinity with the electrolyte 80
solution. Concerning the control over the electrolyte Untreated
decomposition, Endo et al. have reported that a dia- Capacity (mAhg -1 ) 60 RT
mond-like carbon coating can control the decomposi- 373K
tion [11]. However, in this case, the resistivity in the 40 473K
cathode increased significantly. Surface fluorination
can be used to improve the stability of the cathode 20
active material surface, while preciously controlled 0 10 20 30 40 50
surface fluorination can achieve some advantage [12]. Cycle number (times)
Fig. 36.4 shows the effect of the surface fluorination
of LiMn O , by using F gas for its cycling ability. Figure 36.4
2 4 2
During surface fluorination under 1.3 kPa F at room Change in the discharge capacity along the cycle number
2
temperature, the discharge capacity increased by 5% of LiMn O after fluorinating the surface by F gas.
4
2
2
and the loss of discharge capacity after 50 cycles was
limited to just 2%, while the untreated sample exhib- investigation of other carbon materials as the anode
ited a 13% loss in discharge capacity. On the other material for the lithium ion battery to boost capacity.
hand, excess fluorination such as less than 1.3kPa F 1
2 To date, a charge/discharge capacity of 340 mA h g
at 200 C caused a particular decrease in discharge has been achieved, which is almost the theoretical
capacity and an increased loss of discharge capacity capacity for the C/C Li system. In the case of a carbon
6
after 50 cycles. Since excess fluorination may result anode of a lithium ion battery, the electrolyte is decom-
in resistive LiF film formation on the LiMn O parti-
2 4 posed to generate a Solid Electrolyte Interface (SEI) in
cle, it is important to introduce fluorine only on the the first charge process, which plays an important role
surface and control its amount preciously. Hybrid in maintaining stable charge/discharge cycling, so
coating with carbon and fluorine has also been pro- various approaches have been implemented to prepare
posed and investigated by Takashima et al. It has been an SEI suitable for charge/discharge cycling by sur-
reported that this type of surface modification may face treatment and combination of the species in the
have a synergic effect of improving the electrochemi- electrolyte. Surface fluorination [14–16], surface
cal properties of the LiMn O particle [10,13].
2 4 oxidation [17–19], heat treatment under high vacuum
[20], metal coating [21], carbon coating [22–24] and
4. Anode of the lithium ion battery so on have been reported as methods to modify the
surface of the anode active materials. Treatment with
The anode active material used in the lithium ion bat- an oxidative process may affect the morphology near
tery is generally carbon material. Amorphous carbon the surface, while heat treatment under a high vacuum
was first applied to the practical cell, followed by may have the effect of removing the oxide film on the
573