Page 600 - Book Hosokawa Nanoparticle Technology Handbook
P. 600
APPLICATIONS 36 DEVELOPMENT OF A HIGH-PERFORMANCE SECONDARY BATTERY
thickness covered the particles uniformly and was rel- means of solving the aforementioned internal resist-
atively porous. Meanwhile, the dissolution of the ance problem. In their method, CoO, Co(OH) or Co
2
manganese from the alloy was limited to less than one powders were added to the cathode. These cobalt
tenth while no particular loss of discharge capacity compounds were dissolved into the alkaline elec-
was observed. Consequently the Ni–PTFE composite trolyte, whereupon the cathode was covered with
film works as an anticorrosive film and a catalyst to Co(OH) . During the oxidation process (charge
2
promote the redox reaction of hydrogen. It appears process), this Co(OH) was converted to CoOOH,
2
important to design the nano structure of Ni–PTFE which has high electric conductivity, allowing a very
composite film on the anode active material to effective electron conduction path network to be pre-
improve the electrochemical properties of hydrogen pared. Since CoOOH is stable within the potential
storage. range where Ni(OH) works as a cathode, this net-
2
work is retained during the charge/discharge process.
A usage rate of 95% for the cathode active material
2. Cathode of the nickel hydrogen battery
was achieved by this method, as shown in Fig. 36.3
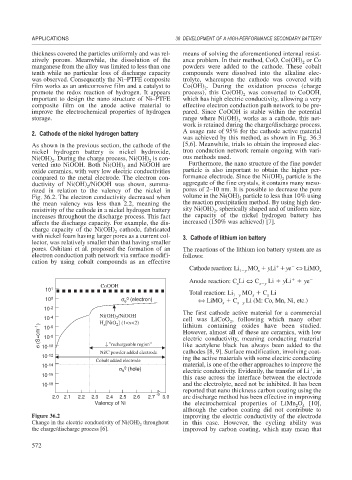
As shown in the previous section, the cathode of the [5,6]. Meanwhile, trials to obtain the improved elec-
nickel hydrogen battery is nickel hydroxide, tron conduction network remain ongoing with vari-
Ni(OH) . During the charge process, Ni(OH) is con- ous methods used.
2
2
verted into NiOOH. Both Ni(OH) and NiOOH are Furthermore, the nano structure of the fine powder
2
oxide ceramics, with very low electric conductivities particle is also important to obtain the higher per-
compared to the metal electrode. The electron con- formance electrode. Since the Ni(OH) particle is the
2
ductivity of Ni(OH) /NiOOH was shown, summa- aggregate of the fine crystals, it contains many meso-
2
rized in relation to the valency of the nickel in pores of 2–10 nm. It is possible to decrease the pore
Fig. 36.2. The electron conductivity decreased when volume in the Ni(OH) particle to less than 10% using
2
the mean valency was less than 2.2, meaning the the reaction precipitation method. By using high den-
resistivity of the cathode in a nickel hydrogen battery sity Ni(OH) , spherically shaped and of uniform size,
2
increases throughout the discharge process. This fact the capacity of the nickel hydrogen battery has
affects the discharge capacity. For example, the dis- increased (150% was achieved) [7].
charge capacity of the Ni(OH) cathode, fabricated
2
with nickel foam having larger pores as a current col- 3. Cathode of lithium ion battery
lector, was relatively smaller than that having smaller
pores. Oshitani et al. proposed the formation of an The reactions of the lithium ion battery system are as
electron conduction path network via surface modifi- follows:
cation by using cobalt compounds as an effective
Cathode reaction: Li 1 y MO yLi ye ⇔ LiMO x
x
Anode reaction: C Li ⇔ C x y Li yLi ye
x
CoOOH
10 1
Total reaction: Li 1 y MO C Li
x
x
0
10 0 σ (electron) ⇔ LiMO C x y Li (M: Co, Mn, Ni, etc.)
e
x
10 -2
The first cathode active material for a commercial
Ni(OH) /NiOOH
10 -4 2 cell was LiCoO , following which many other
2
H [NiO ] (1<x<2) lithium containing oxides have been studied.
2
σ (S•cm -1 ) 10 -8 ↓ rechargeable region” However, almost all of these are ceramics, with low
x
-6
10
electric conductivity, meaning conducting material
”
-10
10
cathodes [8, 9]. Surface modification, involving coat-
Ni/C powder added electrode like acetylene black has always been added to the
10 -12 ing the active materials with some electric conducting
Cobalt added electrode
10 -14 material, is one of the other approaches to improve the
σ (hole) electric conductivity. Evidently, the transfer of Li , in
0
h
10 -16
this case across the interface between the electrode
10 -18 and the electrolyte, need not be inhibited. It has been
reported that nano thickness carbon coating using the
2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 3.0 arc discharge method has been effective in improving
Valency of Ni the electrochemical properties of LiMn O [10],
2
4
although the carbon coating did not contribute to
Figure 36.2 improving the electric conductivity of the electrode
Change in the electric conductivity of Ni(OH) throughout in this case. However, the cycling ability was
2
the charge/discharge process [6]. improved by carbon coating, which may mean that
572